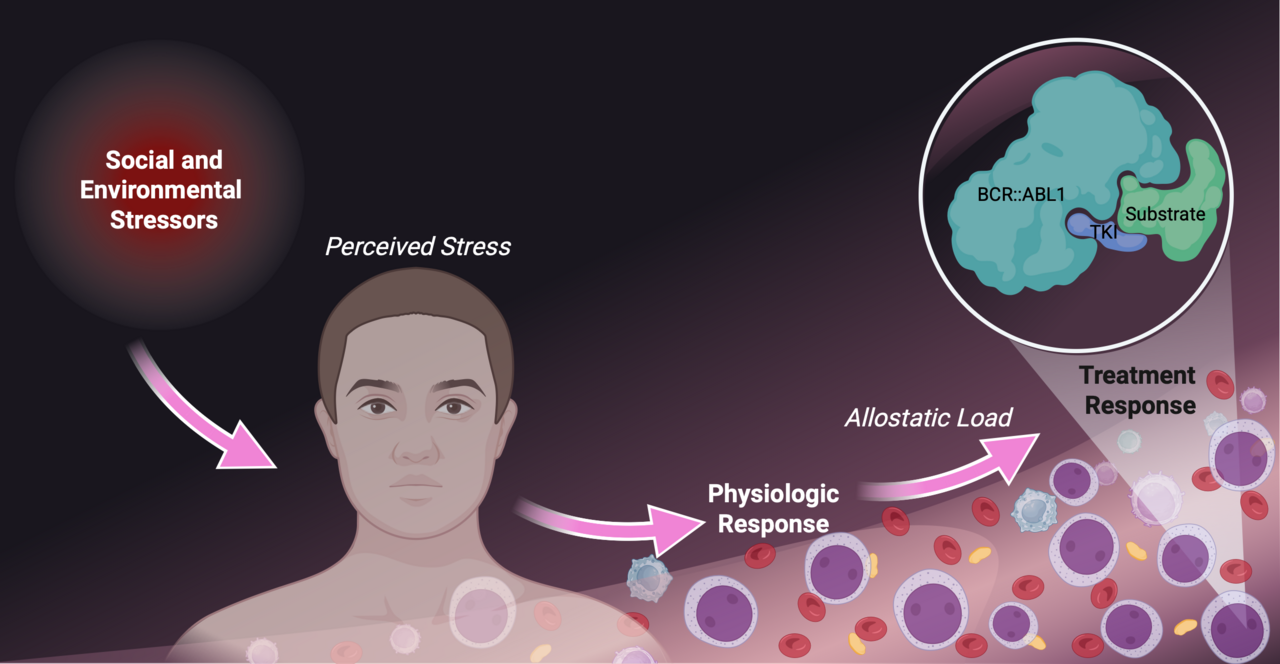
Why do patients with chronic myeloid leukemia respond so differently?
Chronic myeloid leukemia is often hailed as a success story in cancer research. The discovery of the disease-driving BCR::ABL1 gene rearrangement and the development of tyrosine kinase inhibitors have dramatically extended survival, allowing many patients to expect a near-normal life span.
Yet treatment outcomes vary widely. While some achieve rapid and deep molecular responses and may even attempt treatment-free remission, others experience resistance or significant side effects, necessitating sequential use of multiple tyrosine kinase inhibitors over their lifetime.
While leukemia biology and drug mechanisms explain part of this variation, they do not tell the full story. This raises a critical question: what other factors influence how patients respond to treatment?
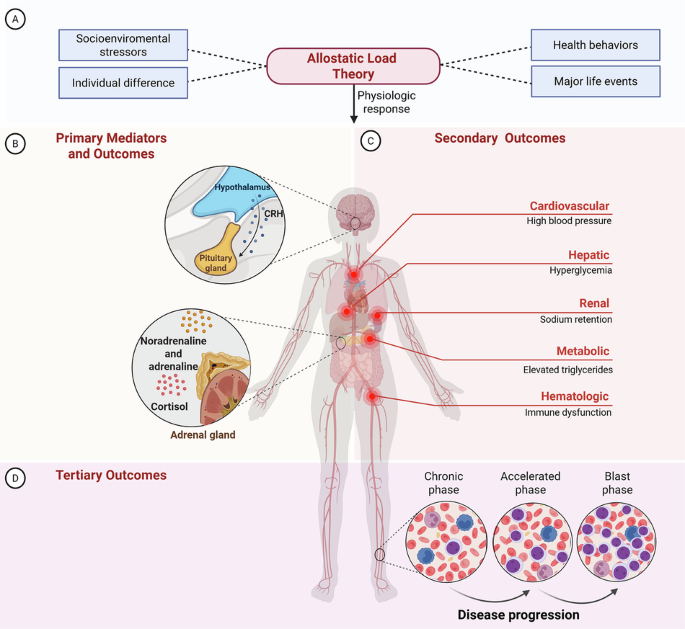
Allostatic load: making stress measurable.
Our research explores this question through the lens of allostatic load, the cumulative physiological toll of chronic stress. Allostatic load reflects repeated activation of stress-response systems, including hormones like cortisol and adrenaline, which over time disrupt multiple organ systems. Crucially, allostatic load can be measured objectively using biomarkers already collected in routine care.
For patients with chronic myeloid leukemia, stress is pervasive: lifelong therapy, frequent monitoring, side effects, financial pressures, and healthcare access challenges all add to the burden. Using 21 biomarkers across five physiological systems, we assessed the allostatic load index in a cohort of patients with chronic myeloid leukemia.
Key findings from our study:
- Social disadvantage amplifies stress. Patients with high allostatic load were more likely to live in low-income areas and engage in less physical activity.
- High allostatic load predicts poorer outcomes. Increased allostatic load index significantly reduced the odds of achieving optimal molecular response.
- Progression and mortality cluster in high-allostatic load patients. All disease progression and deaths occurred among patients with high allostatic load.
- Advanced-phase disease leaves lasting stress imprints. Patients with prior accelerated or blast-phase had persistently higher allostatic load, even after returning to chronic phase.
Clinical impact: why this matters.
Our findings suggest that allostatic load is not just a background measure, but a potentially actionable tool in chronic myeloid leukemia care.
- For clinicians: Allostatic load could help identify patients at higher risk of treatment failure or disease progression, guiding closer molecular monitoring, earlier therapy adjustments, or referral to supportive care services such as stress management programs, physical activity interventions, or cardio-oncology care.
- For patients: Recognizing the role of stress biology emphasizes that successful treatment extends beyond controlling leukemia cells. Incorporating strategies to reduce stress, improve lifestyle, and increase access to supportive resources may lower physiological burden, enhance adherence to TKIs, and improve quality of life.
- For health systems: Integrating molecular biology with social context, including access to wellness resources, could help reduce disparities in treatment outcomes, particularly in underserved populations where social disadvantage amplifies biological stress.
Looking ahead.
Chronic myeloid leukemia care continues to improve, with next-generation TKIs, sensitive molecular monitoring, and increasing success in treatment-free remission. Yet, stress biology and social context remain under-recognized drivers of outcome variability. True precision medicine must extend beyond genomics to consider these factors.
Next questions include which contributors to allostatic load can be modified, whether reducing stress improves disease outcomes, and how such measures can be integrated into research and clinical care. Future trials should evaluate stress biology and social determinants alongside genomic testing to achieve patient-centered care.
We invite clinicians, researchers, and patients to join this conversation. Together, we can reimagine CML care, where managing leukemia means addressing not only the malignancy itself, but also the unseen weight of stress that shapes every patient’s journey.
Figure Created in BioRender. Miranda, M. (2025) https://BioRender.com/ogi0giq
Follow the Topic
-
Leukemia

This journal publishes high quality, peer reviewed research that covers all aspects of the research and treatment of leukemia and allied diseases. Topics of interest include oncogenes, growth factors, stem cells, leukemia genomics, cell cycle, signal transduction and molecular targets for therapy.

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in