The implications of the contamination of Arctic ice samples to future space missions to Europa and Enceladus
Published in Astronomy
Text written by Lígia F. Coelho, João Canário and Zita Martins
Europa and Enceladus are two icy moons from our solar system identified as ocean worlds due to the presence of a liquid ocean under, and within, their icy surface. Several concepts for future lander missions to these moons have been developed, and these space missions will need to either analyse potential geysers, or drill and sample a thick layer of ice to eventually reach the interface water. But can this be achieved in a clean way, with no risk of forward contamination (i.e., contamination from Earth microbes)? This is the golden question of planetary protection applied to the exploration of icy moons. The Committee of Space Research (COSPAR) recommends that the study of methods of bioburden reduction for these missions should reflect the type of environments found on Europa or Enceladus, focusing on Earth organisms most likely to survive on these moons, such as cold and radiation tolerant organisms. In order to achieve this, our team collected samples during a field trip in the Arctic (Figure 1), which has an environment that mimics some of the conditions of these icy moons.

The Arctic, the Polar Region located at the northernmost part of Earth, is a unique area among Earth's ecosystems. The diversity of the northern landscapes has similar features to what appears in Jovian and Saturnian moons, such as Europa and Enceladus: from the polar deserts in high latitudes to the immense areas of glacier and sea ice. In addition, the Arctic has impressive biodiversity living in extreme conditions, in particular very cold temperatures. In this work the Hudson Bay near the Cree and Inuit communities of Whapmagoostui-Kuujjuarapik (Canada) were chosen for our fieldwork in March 2019. This subarctic area at approximately 55º16’ latitude N is characterised by short, cold and windy winters with temperatures ranging (in average) between -25ºC and -13ºC. The shore of Hudson Bay, a vast sea that is covered by ice usually between November and May, is also surrounded by rivers and lakes of distinct origins. This bay and the mouth of the Great Whale River allowed us to sample ice and water with different salinity (in some cases agreeing with the salinity measured at Enceladus by the Cassini spacecraft), and with varying river influence, which is reflected in their chemical and biological composition. Therefore, these icy landscapes were considered an ideal planetary field analogue.
When studying recent terrestrial ice, contamination is critical, and sources are mainly due to sample collecting tools such as ice corers, handling, and transportation. Other contamination sources, such as air microbes are not expected to be present in icy worlds, which have a very thin or no atmosphere. In addition, procedures will be automated in future lander space missions, representing points of contact between man-made equipment coming from Earth and extraterrestrial samples. Sterilisation methods used for space equipment cannot be directly applied to ice samples, as these would modify or destroy the content of the icy samples. Furthermore, complete sterilisation is a theoretical limit, and thus investment in contamination monitoring using background controls should be considered. The decontamination methodology for the ice core surface was mechanical (i.e., removal of external ice core layers) (Figure 2, top). Our results show that mechanical removal of the external ice core layers is effective in reducing bioburden and does not destroy the natural biota present in the interior of the ice cores.
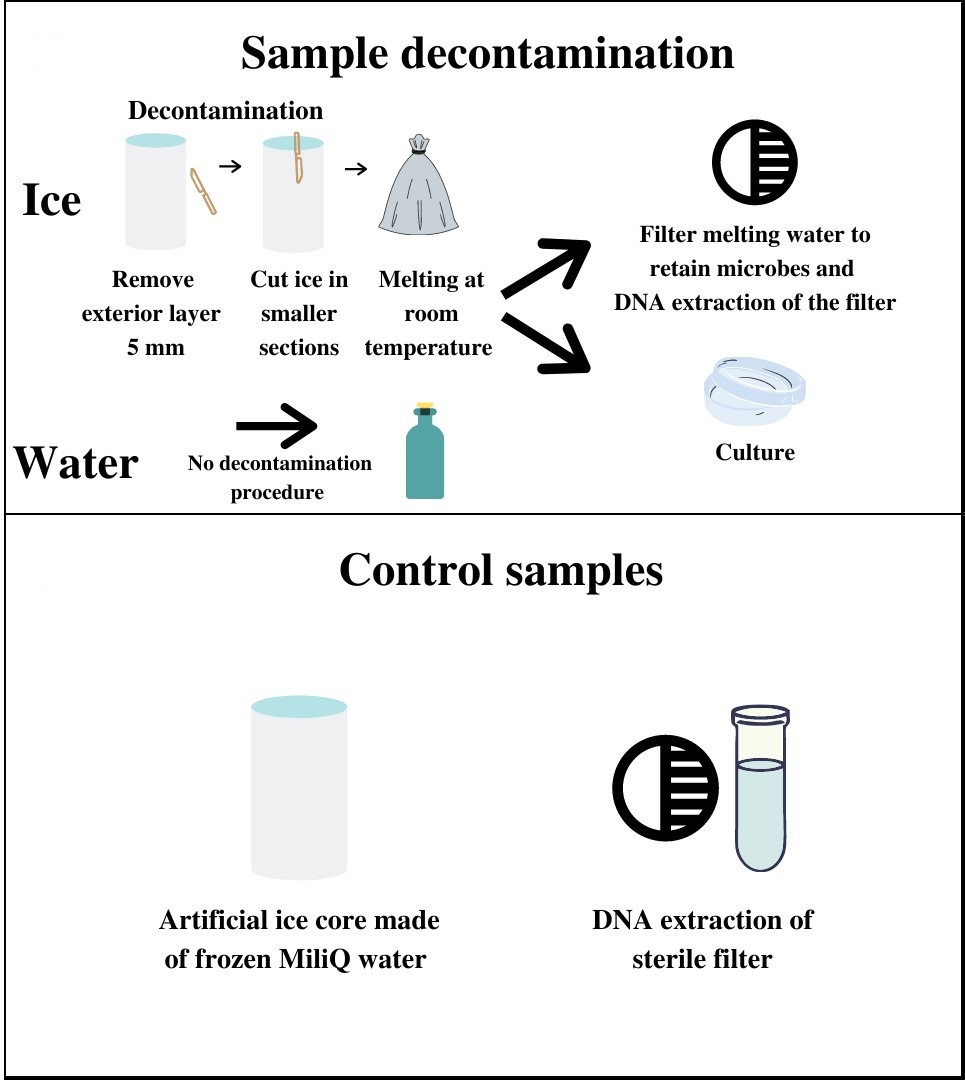
Figure 2 - (Top) Sampling decontamination procedure and following processing preceding culture-dependent and culture-independent analysis. (Bottom) Control samples: an artificial sterile ice core made in the laboratory (processing control), and a clean filter inside a clean microtube used as a control for downstream DNA analysis (DNA extraction control). Figure taken and adapted from Coelho et al. 2022.
In addition, we constructed background controls: an artificial ice core made of sterile MiliQ water (the Processing Control) and a DNA extraction control sample (the DNA-extraction Control - or DC) (Figure 2, bottom). We used both culture-dependent and -independent techniques, such as 16S rRNA gene amplicon sequencing of metagenomic DNA samples, accessing several levels of visible and quantifiable microbial prokaryotic contamination. We identified the contaminating microorganisms of the present study using an established ribosomal marker database to understand the astrobiology relevance of the contaminants. Such a decontamination protocol is suitable for the design of life-detection experiments on planetary field analogues of Europa and Enceladus, targeting ice cores that may serve as a proxy for habitats of putative extraterrestrial communities.
Guaranteeing the protection of solar system celestial bodies from terrestrial contamination is a crucial point in in-situ and sample return missions to icy moons, to be able to correctly identify putative extraterrestrial microorganisms. In particular, forward contamination in future space missions is a concern, with the NASA Procedural Requirements (NPR) defining the forward contamination in ocean worlds as “the introduction of a single viable terrestrial microorganism into a liquid–water environment”. There is an increasing interest in the icy moons of the solar system, with the announcement of the Enceladus Orbilander, proposed in the 2023-2032 Planetary Science Decadal Survey as a NASA Flagship mission, with potential landing in 2050s to study this moon for evidence of life. Furthermore, during the 2021 ESA Intermediate Ministerial Meeting it was announced that the Voyage 2050 Senior Committee has chosen the future science mission themes, with ESA’s large-class science missions for the timeframe of 2035 to 2050 to focus on the moons of the giant solar system planets. Therefore, the next decades will need straightforward protocols to decontaminate icy samples that will be analysed by future space missions.
Follow the Topic
-
Scientific Reports

An open access journal publishing original research from across all areas of the natural sciences, psychology, medicine and engineering.
Related Collections
With Collections, you can get published faster and increase your visibility.
Obesity
Publishing Model: Hybrid
Deadline: Apr 24, 2026
Reproductive Health
Publishing Model: Hybrid
Deadline: Mar 30, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in