The ins and outs of nuclear β-arrestin2 on Mdm2-p53 signalling
Published in Cancer
Mdm2 antagonizes tumour suppressor p53 function. Targeting the Mdm2-p53 interaction represents an attractive approach for the treatment of cancers with functional p53. Investigating mechanisms that impact the Mdm2-p53 signalling axis is therefore of fundamental importance.
The β-arrestins (β-arr1 and β-arr2) are scaffold proteins, initially discovered for the roles they play in the regulation of G protein-coupled receptors. Via their scaffolding properties, the β-arrs also dynamically control the activity and/or subcellular distribution of key intracellular signalling proteins including Mdm2. Whereas β-arr1 is located both in the nucleus and cytoplasm, β-arr2 displays an apparent cytoplasmic distribution. This is due to constitutive ejection of β-arr2 from the nucleus by a leptomycin B-sensitive pathway, driven by a nuclear export signal (NES) that is absent in β-arr1. β-arr2 is also actively imported into the nucleus indicating that it undergoes constitutive nucleocytoplasmic shuttling. β-arr2 shuttling displaces Mdm2 from the nucleus to the cytoplasm, resulting in increased p53 signalling and cell cycle arrest. In contrast to the well characterized nuclear export mechanism of β-arr2, knowledge on the entry mechanism(s) of β-arr2 into the nucleus and functional impact on Mdm2-p53 signalling remains incomplete.
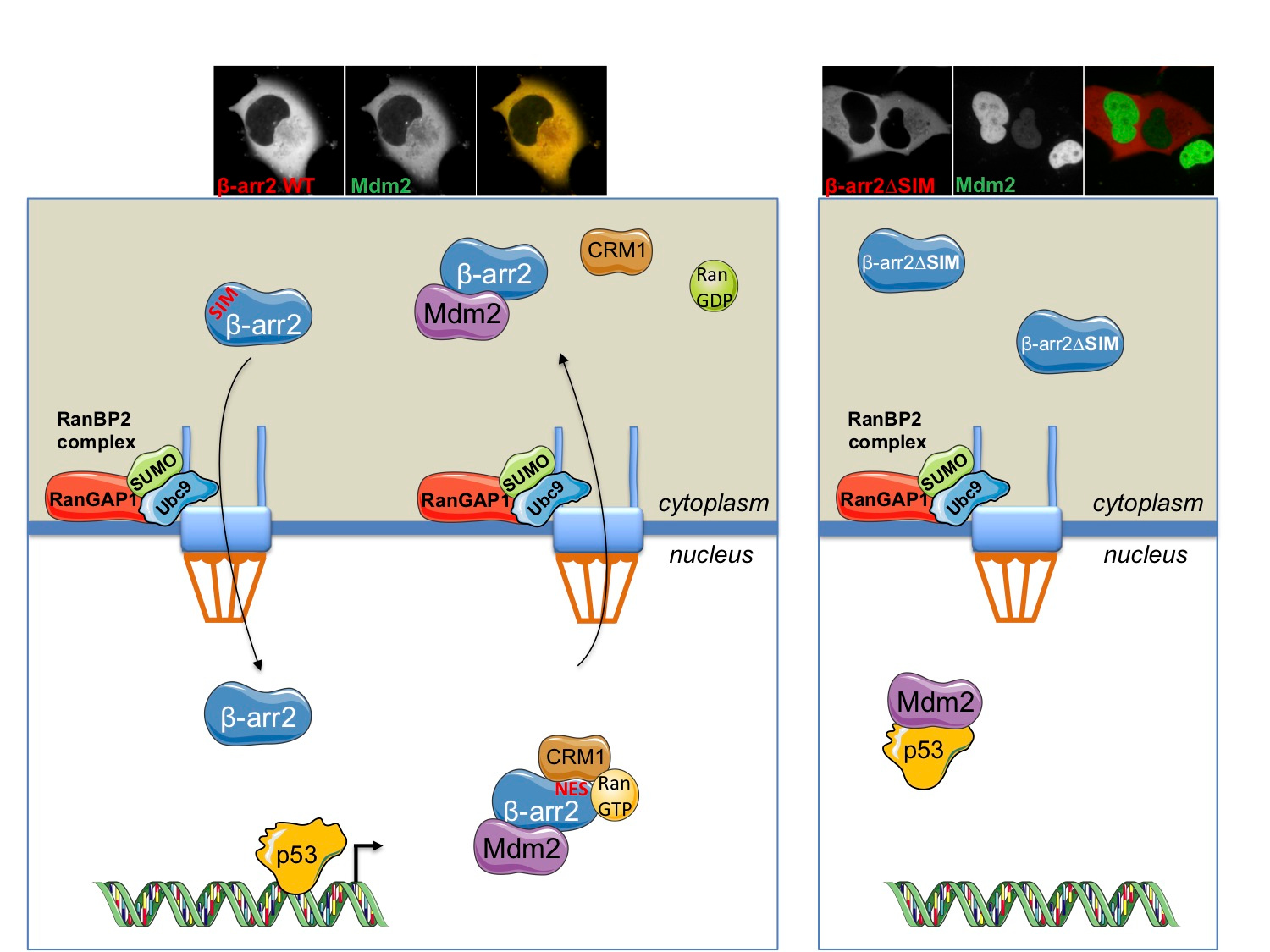
We set out to determine if small ubiquitin-like modifier (SUMO) could be involved in the coordination of β-arr2 nucleocytoplasmic shuttling. SUMOylation is a key regulatory post-translational modification that impacts the activity and localization of protein targets. In addition to SUMOylation sites for covalent conjugation on lysine residues, SUMO interaction motifs (SIMs) exist, composed of a short stretch of hydrophobic residues that mediate non-covalent interaction between SUMO and SIM-containing proteins. Using various in vitro, in silico and cell-based approaches we characterized both a SUMOylation site and SIM in β-arr2. Whereas SUMOylation was not required for nuclear import, we found that the SIM on β-arr2 was. The SIM promotes association of β-arr2 with the multimolecular RanBP2/RanGAP1-SUMO nucleocytoplasmic transport hub that resides on the cytoplasmic filaments of the nuclear pore complex (Fig. 1). Depletion of the RanBP2/RanGAP1-SUMO complex resulted in defective nuclear import of β-arr2 demonstrating its functional importance in β-arr2 cytonuclear trafficking.
We next assessed the function of the β-arr2 SIM on Mdm2-p53 signalling. Due to defective nuclear import, a β-arr2∆SIM mutant lost the ability to titrate Mdm2 from the nucleus to the cytoplasm (Fig. 1). In non-small cell lung carcinoma and breast tumour cell lines the enhancing effect of β-arr2 on p53 signalling was also lost with the β-arr2∆SIM mutant. Taken together our results indicate that a β-arr2 SIM nuclear entry checkpoint, coupled with active nuclear export, regulate its cytonuclear trafficking to control the Mdm2-p53 signalling axis (Fig. 1). Future studies will be required to determine if β-arr2 cytonuclear shuttling goes awry in cancer.
Follow the Topic
-
Oncogene

This journal aims to make substantial advances in our knowledge of processes that contribute to cancer by publishing outstanding research.




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in