The interplay between virulence mechanisms is a game changer for fungal pathogenesis
Published in Microbiology

Cryptococcus neoformans is one of four fungal species recently assigned by the World Health Organization to the most dangerous ‘critical priority group’ of fungal pathogens 1. Infections are often fatal in immunocompromised individuals, causing an estimated 112,000 deaths annually and accounting for 19% of AIDS-related mortality 2. Research in our lab focuses on traits of C. neoformans, like its polysaccharide capsule and melanin production, that promote survival of this deadly pathogen inside the human body with the goal of exploiting these virulence mechanisms as targets for vaccine development or treatment strategies. Our current investigation has revealed an interdependent relationship between two such virulence factors of C. neoformans, ammonia production by urease and melanization of the cell wall. Collusion between individual factors is a game changer as it provides the fungal pathogen with a means of quickly adapting to changing environments within the human host, thereby adding a layer of complexity to predicting the outcome of infection.
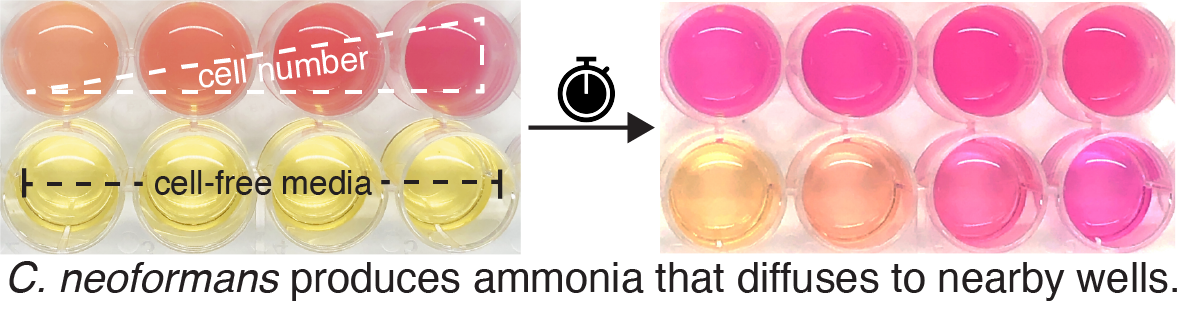
This project, as many discoveries in science, began with a serendipitous observation. I was titrating cell density to determine the optimal number of C. neoformans cells to use in a urease activity assay, a colorimetric assay based on increasing pH of urea-supplemented media from acidic to alkaline. I set up the assay with increasing numbers of cells in four wells of a multi-well plate, but I also added cell-free media in four adjacent wells to serve as controls for absorbance readings. I was surprised to find that after incubation for 6 hours, not only had the media in wells containing C. neoformans cells changed color from yellow to pink, the media in the cell-free control wells had also changed color, and in a linear fashion with respect to the number of cells in the adjacent well! This was reminiscent of a quorum sensing mechanism, and I started to wonder if ammonia produced by one cell would be able to affect other cells in its surrounding environment.
Having also studied melanin production by C. neoformans, I was aware that polymerization of phenolic precursors to produce melanin was accelerated at alkaline pH so I was intrigued to explore how cells releasing diffusible ammonia might affect the rate of melanin production by nearby cells. I designed an assay in which cells were grown on agar supplemented with melanin precursors in a multi-well plate that also contained either wild-type or urease-deficient cells growing in urea-supplemented media. Pigment production was accelerated in the plate containing wild-type cells producing ammonia compared to the plate with urease-deficient cells and the rate increased linearly with proximity to the source of ammonia. This was an exciting observation as it revealed that a virulence factor expressed in one cell can act at a distance to modulate a different virulence factor in another cell. I remember relating these observations to my co-author, Arturo Casadevall, and how he responded with the famous Louis Pasteur quote, “chance favors only the prepared mind.” As I continued to investigate the effect of ammonia on melanization, I learned that the ammonia-mediated pH increase not only favors the chemical reaction of melanin precursors, but also promotes expression and cell-wall localization of the enzyme, laccase 1, primarily responsible for melanin production by C. neoformans.
The next set of experiments brought the investigation ‘full-circle’ as I discovered that urease activity and ammonia release were decreased for melanized compared to non-melanized cells. Many of the virulence factors expressed by C. neoformans, including urease, are released from the cell in lipid-bound vesicles. Melanin deposition on the cell wall was found to physically impede the release of these extracellular vesicles, thereby limiting urease activity. Thus, urease and melanin production are reciprocally modulated through a feedback mechanism in which ammonia production by urease bolsters melanin synthesis while melanin in turn impedes ammonia production by urease. This was an exciting finding as it revealed an interdependent relationship between two virulence factors in this fungal pathogen that informs our future disease intervention efforts to consider the virulence composite rather than focusing on individual virulence factors in isolation.
 Created with BioRender.com
Created with BioRender.com
Having characterized the interaction between two virulence factors in cell culture, I shifted my focus to look for evidence of interplay between ammonia and melanin production in an animal model of cryptococcal infection. Using a hand-held ammonia meter, I measured ammonia gas in the breath of mice infected with a high titer of urease-positive C. neoformans cells in their lungs. Moreover, lung tissue pH was higher for animals infected with wild-type compared to urease-deficient cells which is in accordance with ammonia production by active urease during infection. Histological examination of infected lung tissue revealed an increased number of melanized cells for mice infected with wild-type compared to urease-deficient C. neoformans and this is consistent with the ammonia-mediated stimulation of melanin production observed in cell culture. Finally, comparing the outcome of intravenous delivery of macrophages carrying non-melanized or melanized cells, I detected higher fungal burdens in the lungs and brains of animals infected with melanized cells. This suggests that melanin confers a survival advantage for C. neoformans in the harsh environment of the phagocytic host cell to promote brain dissemination through a ‘Trojan horse’ mechanism.
This project has underscored for me the importance of careful observation and being open to surprising results as this can be the very seed of a new avenue of investigation. I find the possibility of identifying additional interactions between fungal virulence factors intriguing and I look forward to exploring this in the future.
References:
- World Health Organization. WHO fungal priority pathogens list to guide research, development and public health action. https://www.who.int/publications/i/item/9789240060241 (2022).
- Rajasingham, R. et al. The global burden of HIV-associated cryptococcal infection in adults in 2020: a modelling analysis. Lancet Infect. Dis. 22, 1748–1755 (2022).
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in