The Invisible Threat: Why Micronanoplastics are a Systemic Immune Problem Across All Organ Systems
Published in Earth & Environment and Immunology
The Spark: Why This Review Now?
Over the last few years, research has confirmed that we ingest and inhale thousands of micronanoplastic (MNP) particles daily. Yet, the scientific community often operates in silos—studies on pulmonary effects are disconnected from those on reproductive failure or neurotoxicity.
This fragmentation led us to a crucial question: Is there a common mechanism linking these disparate adverse outcomes? We realized the scientific landscape required a comprehensive synthesis that connected these isolated "islands" of data, defining MNPs as a systemic, immunity-driven health problem.
The Challenge: Mapping the Immune Highway
The task was colossal. We had to synthesize evidence from human, animal, and in vitro models, covering eight major body systems (nervous, endocrine, respiratory, digestive, and others).
The greatest challenge was reconciling the vast differences in MNP data—particles varied in shape, size, material, and concentration across studies. We needed to identify a universal language of toxicity that could explain why such diverse particles lead to such consistently alarming pathologies.
Our Key Finding: Inflammation is the Common Thread
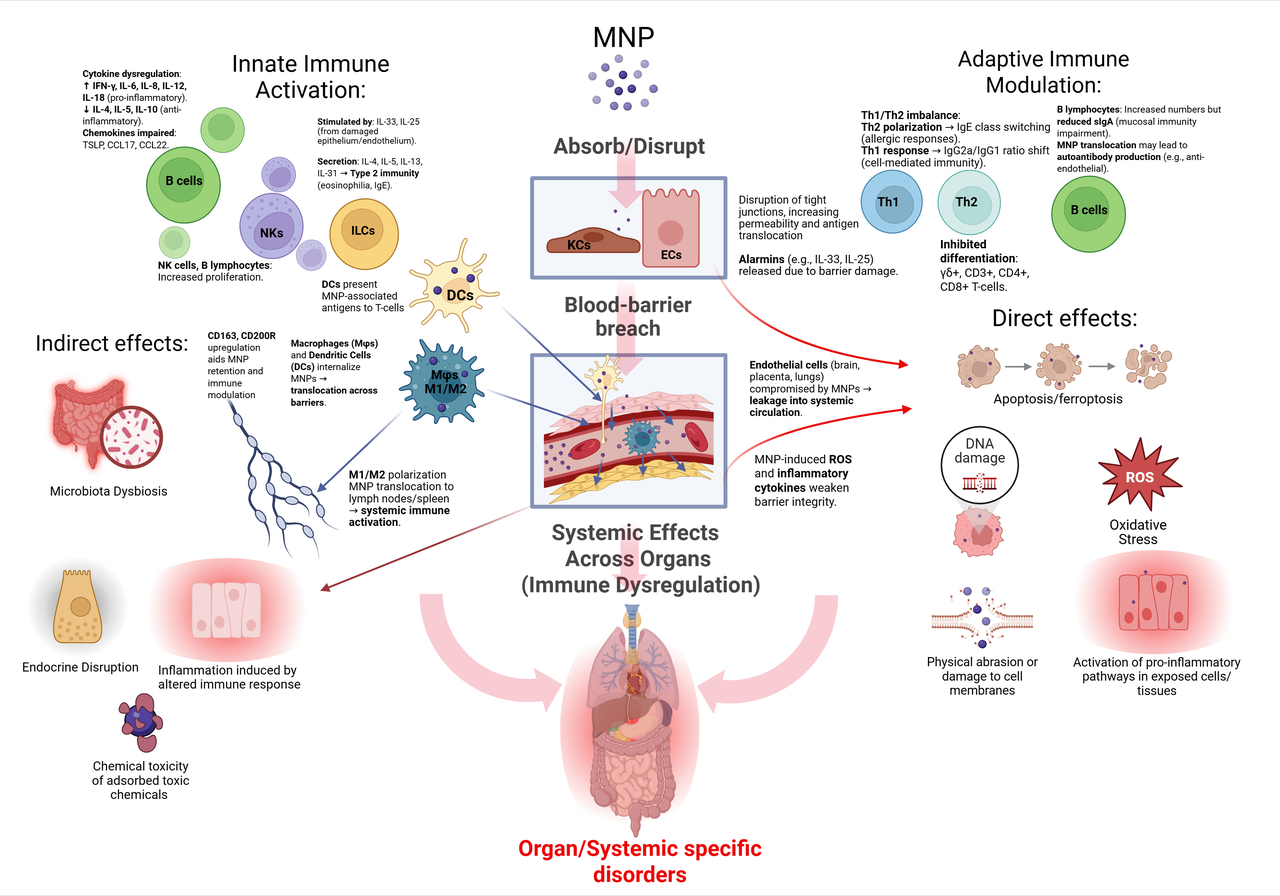
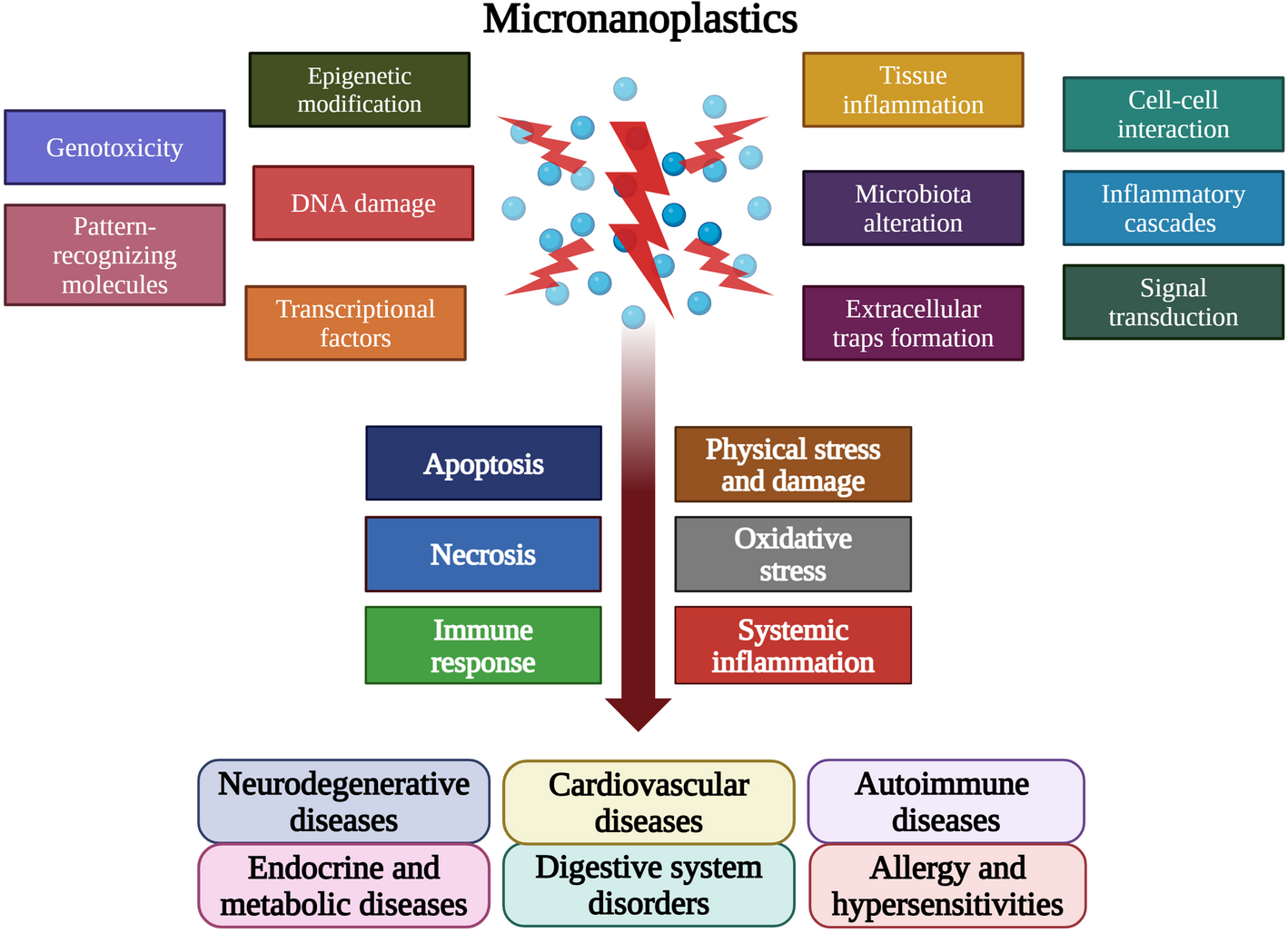
Our analysis revealed that MNPs are not passive, inert particles; they are active disruptors of cellular processes. The core mechanism of their harm is a cascading immune dysfunction, primarily manifesting through two pathways:
-
Oxidative Stress: MNPs induce excessive production of free radicals, damaging cells across all organs.
-
Immune Dysregulation: The particles trigger persistent, low-grade inflammation that can contribute to neuroinflammation, endocrine disruption, and other chronic illnesses.
In essence, we found that the immune system acts as the highway through which MNPs exert their systemic effects across the entire body.
The Research Agenda: What Happens Next?
Our review is not an endpoint; it is a roadmap. It’s a clear call to action for the scientific community. To effectively combat this global threat, standardization is essential. We urge researchers to:
-
Standardize Protocols: Adopt unified testing protocols and MNP characterization metrics to make results truly comparable globally.
-
Focus on Nanoplastics: Increase research on the smallest fraction—nanoplastics—as their capacity to cross biological barriers (like the blood-brain and placental barriers) presents the most significant threat.
-
Transition to Clinical Relevance: Develop clinical intervention and prevention strategies to protect human health from this pervasive environmental danger.
Follow the Topic
-
Discover Medicine

This is a fully open access, peer-reviewed journal that supports multidisciplinary research and policy developments across the fields of medical and clinical science.
Ask the Editor - Immunology, Pathogenesis, Inflammation and Innate Immunity
Got a question for the editor about the complement system in health and disease? Ask it here!
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
Gut Microbiome and Human Well Being
The human gut microbiome plays a critical role in maintaining health and modulating disease processes. Its composition evolves across the lifespan under the influence of diet, lifestyle, aging, and environmental exposures. Disruption of gut microbial balance—known as dysbiosis—has been implicated in the pathogenesis of various chronic conditions, including autoimmune, metabolic, neurological, and inflammatory diseases.
This Collection welcomes submissions that explore the mechanistic, clinical, and translational aspects of gut microbiota in human health, with particular interest in:
The gut–brain, gut–heart, gut–cancer, and gut–joint axes
Gut dysbiosis and its immunological and metabolic consequences
Microbial metabolites and host-microbiome interactions
Microbiome-based diagnostics, therapeutic interventions, and biomarkers
We invite contributions including but not limited to:
Original research articles based on clinical, in vivo or in vitro studies
Reviews summarizing recent advances or proposing new frameworks
Perspective or opinion pieces that highlight emerging concepts or challenges
This Collection aims to enhance our understanding of the gut microbiome's contribution to health and disease and to inspire microbiome-based strategies for improving human well-being.
Keywords: Gut microbiome; Gut-organ-axis; chronic diseases; 16S rRNA; metagenomics
Publishing Model: Open Access
Deadline: Jul 01, 2026
Gender and Ethnic Disparities in Cardiovascular Health and Diseases
The global burden of cardiovascular disease (CVD) is alarming, with profound gender and ethnic disparities. Women are more likely to be underdiagnosed and undertreated, while ethnic minorities, including African Americans, Hispanics/Latinos, American Indians, and those in underserved communities, bear a disproportionate burden of CVD. Socioeconomic factors, inadequate healthcare access, and cultural barriers exacerbate these disparities. These disparities are not only a reflection of biological differences but also encompass a complex interplay of social determinants, healthcare access, and cultural factors. Understanding these dimensions is crucial for developing effective prevention and intervention strategies aimed at mitigating cardiovascular health inequalities. To bridge these gaps, rigorous research, robust data collection, and effective dissemination of findings are imperative in identifying these drivers.
This Collection will provide a platform for experts to share their research, insights, and solutions to address these pressing issues. We believe this Collection will contribute significantly to the ongoing conversation on health disparities and inform strategies to reduce the burden of CVD. We invite researchers and scientists to submit their original work to our Collection focused on gender and ethnic disparities in cardiovascular health and diseases. This Collection will contribute significantly to our understanding of the complex relationships between gender, ethnicity, and cardiovascular health outcomes and provide valuable insights into the drivers of these disparities and inform strategies for improvement.
Topics of interest include, but are not limited to:
- Gender differences in cardiovascular disease prevalence
- Ethnic disparities in treatment outcomes
- Sociocultural influences on cardiovascular health
- Impact of social determinants on heart disease risk
- Community-based interventions for at-risk populations
Keywords: Cardiovascular Disease Disparities, Gender and Ethnic Disparities in Health, Healthcare Access and Outcomes, Cardiovascular Health Equity, Racial and Ethnic Disparities in Cardiovascular Disease
This Collection supports and amplifies research related to SDG 3.
Publishing Model: Open Access
Deadline: Apr 14, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in