
The human gut is colonized by a diverse community of microbes that exhibit remarkable differences in composition between healthy and diseased individuals. For instance, people with inflammatory bowel disease (IBD) and obesity, two chronic diseases affect around 20% of the population worldwide, have been shown to have gut microbial communities that differ from those of healthy individuals.
Over the past 10 years, differential abundances of microbes in health and disease have been well characterized and validated by artificial in vivo and in vitro models. Nevertheless, we argue that the diverse microbial communities in our gut make up a complicated ecosystem in which microbes can exchange or compete for nutrients, signaling molecules, or immune-evasion mechanisms through ecological interactions that are far from fully understood. Enthusiasm has thus been rising to decipher these microbial interactions in order to detect key microbes in health and disease.
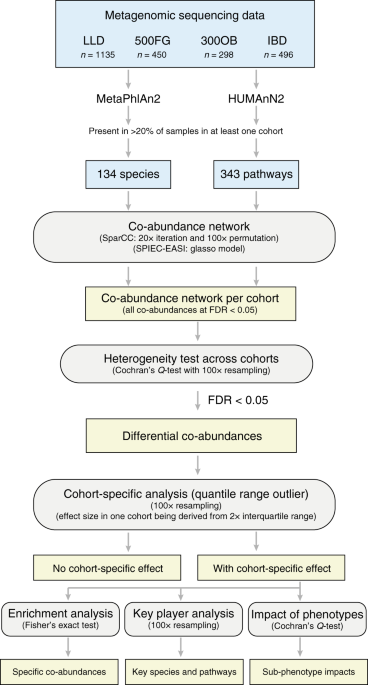
It is currently not possible to construct a humangut microbiota to study microbial interactions in health and disease as many microbes are not yet culturable. However, the gut microbial composition profile generated by shotgun metagenomics sequencing of large human cohorts does allow us to make inferences about microbial interactions in silico by assessing their co-abundance relationship (i.e., correlation of microbial abundance between microbes) and further comparing their differences between health and disease.
To look at these kinds of relationships, we compared the gut microbiome of 2,379 participants from four cohorts in the Netherlands: an inflammatory IBD cohort, an obese cohort and two population-based cohorts (Lifelines-DEEP and 500FG). By constructing and comparing microbial co-abundance networks, we observed that the strengths of 39% of microbial species co-abundances and 64% of microbial pathway co-abundances varied significantly between cohorts. In addition, hundreds of microbial co-abundance relationships showed IBD- and obesity-specific effects that could replicated in independent cohorts. Moreover, we identified key microbes that potentially dominate the diseased gut microbial ecosystem, e.g. Escherichia coli, Oxalobacter formigenes and Actinomyces graevenitzii for IBD.

Our study shows that microbial dysbiosis in disease may not only be driven by differences in microbial abundance level but also by shifts in microbial interactions that are mirrored in co-abundance analyses, which extends our current knowledge about the role of the microbiome in disease. In particular, the disease-specific microbial interactions we identified provide further insights into functional dysbiosis in IBD and obesity.
For details, please see our manuscript published in Nature Communications (2020) 11: 4018. https://www.nature.com/articles/s41467-020-17840-y
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in