The paradox of macrolide resistance in Pseudomonas aeruginosa
Published in Microbiology
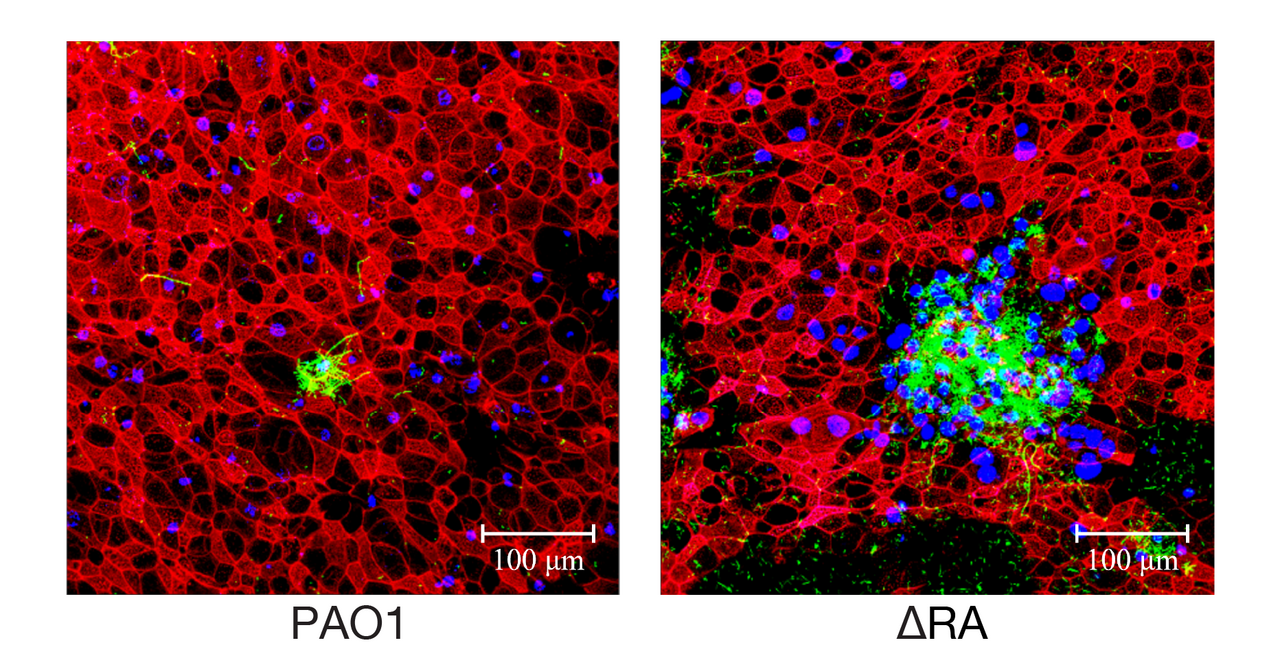
Macrolides are a class of antibiotics widely used to treat bacterial infections, especially those caused by Gram-negative pathogens. They are, however, also widely used to treat chronic airway infections caused by the Gram-negative bacterium Pseudomonas aeruginosa.
Despite their widespread use, the effectiveness of macrolides against Pseudomonas aeruginosa, a common and stubborn pathogen in these conditions, has been questioned. P. aeruginosa is known for its high intrinsic resistance to many antibiotics, including macrolides, primarily due to its high minimum inhibitory concentration (MIC) in standardized culture media. This resistance has led to the assumption that macrolides are ineffective against P. aeruginosa, resulting in the exclusion of macrolides from routine antibiotic susceptibility testing for this pathogen. Consequently, even though macrolides are frequently prescribed to patients with chronic P. aeruginosa infections, their potential effectiveness and the mechanisms of resistance have not been thoroughly investigated.
Our recent study, challenges this long-held assumption by demonstrating that macrolides can be effective against P. aeruginosa in an air-liquid interface (ALI) infection model, which closely mimics human airway conditions. This model allows for a more accurate assessment of antibiotic efficacy in a setting that replicates the complex environment of the human respiratory tract. Furthermore, we uncover the genetic basis of this acquired macrolide resistance, identifying specific mutations in the ribosomal proteins uL4 and uL22 that confer resistance. These findings have significant implications for the clinical management of P. aeruginosa infections and highlight the need for vigilant resistance monitoring.
Key Findings
-
Macrolides as Effective Antibiotics: Contrary to the prevailing belief, our research demonstrates that macrolides can effectively combat P. aeruginosa in an air-liquid interface (ALI) infection model, which closely mimics human airway conditions. This finding is pivotal for patients with cystic fibrosis (CF) and primary ciliary dyskinesia (PCD), who often receive macrolide treatment.
-
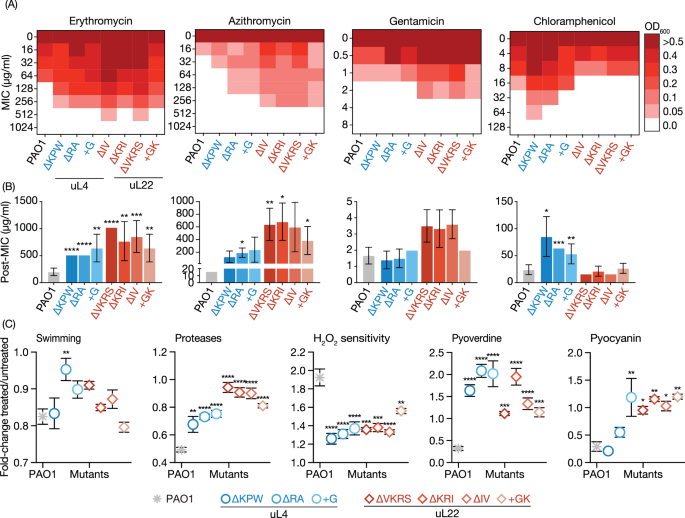
Ribosomal Mutations and Resistance: We identified specific mutations in the uL4 and uL22 ribosomal proteins that confer resistance to macrolides. These mutations necessitate higher antibiotic concentrations to achieve the desired therapeutic effects, highlighting the need for vigilant resistance monitoring.
-
Collateral Effects of Mutations: Interestingly, these ribosomal mutations also lead to reduced bacterial growth rate, virulence, and pathogenicity, even in the absence of antibiotics. This dual effect suggests a complex interplay between antibiotic resistance and bacterial fitness.
Implications for Clinical Practice and Future Directions
Further research is needed to explore the long-term implications of ribosomal mutations on bacterial behavior and patient outcomes. Additionally, integrating advanced infection models that include immune system components could provide deeper insights into the host-pathogen interactions and resistance mechanisms. Comprehensive and conclusive analyses are required to update the standard of care guidelines for the utilization of macrolides in managing long-term chronic infections. This will ensure the delivery of optimal patient care while mitigating the development of antibiotic resistance.
Conclusion
This study marks a significant step forward in understanding macrolide resistance in P. aeruginosa. By unraveling the genetic and phenotypic changes associated with ribosomal mutations, we pave the way for more effective treatment strategies and improved patient care.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in