The right model for the right virus
Published in Microbiology
40 years after the initial pandemic, Human Immunodeficiency Virus 1 (HIV-1) is still a huge global problem with 1.5 million new infections in 2021. Currently, more than 38 million people are living with HIV-1/Aids and despite treatment options, HIV-1 is still one of the leading causes of death in sub-Saharan Africa, particularly for young women. Infection with HIV-1 primarily occurs during sexual contact and the genital mucosa is one of the first tissues to encounter HIV-1. During heterosexual intercourse, women are at greater risk to become infected with HIV-1 than their male peers. Inflammation of the vaginal mucosa and bacterial co-infections (i.e. STDs) are main factors contributing to HIV-1 infection risk. It is therefore paramount to understand how an inflammatory environment and accompanying immune responses influence the risk of an HIV-1 infection in women.
HIV-1 is a notoriously difficult virus to study in vivo due to its adaptation to human hosts. The most commonly used animal models are humanized mice or non-human primates that both come with restrictions. Instead of animal models, HIV-1 infection can also be studied in human tissues. In particular when studying transmission, mucosal tissues constitute an important virus entry site and therefore mucosal explants models are very appropriate to study early events.
In this context, we have used human vaginal mucosa explants to study what happens during initial HIV-1 contact and subsequent infection. Our focus pointed towards early HIV-1 target cells and how these are influenced by inflammation and co-infection. We receive vaginal tissues from women undergoing vaginal prolapse surgery and either infect the vaginal tissue explants or isolate immune cells to investigate HIV-1 infection. The ex vivo vaginal explant model enabled us to study the interaction of HIV-1 with important human target cells in steady state as well as inflammatory environments.
One of the main targets for HIV-1 are Langerhans cells (LCs). These cells are part of the immune system and patrol surface tissues like the vaginal mucosa to spot and deal with incoming pathogens. In the past, we and others could show that LCs isolated from human skin are important in the defense against HIV-1 as LCs efficiently degrade HIV-1 in special organelles called Birbeck granules.
Similar to what we observed in skin, LCs are also present in the vaginal mucosa where LCs populate the outer epithelial layer and are among the first cells to interact with HIV-1 during sexual contact. Importantly, LCs can be present in two different states: immature and activated. Their activation state determines whether LCs prevent or perpetuate HIV-1 infection. Immature LCs are highly efficient protectors against HIV-1 through capture and subsequent degradation of viral particles. Activated LCs however become infected by HIV-1, transmit the unharmed virus to T cells in nearby lymph nodes and hence contribute to further infection and HIV-1 dissemination.
LCs isolated from vaginal mucosa shortly after surgery are in an immature state and are highly expressing CD1a and langerin, similar to what we observed in skin LCs. Interestingly however, we discovered that vaginal LCs express a wider range of Toll-like receptors than their epidermal counterparts. These included the bacterial-sensing and viral-sensing receptors TLR2 and TLR4, suggesting that vaginal LCs are activated by different infections. With our vaginal explant model, we were able to stimulate immature LCs with various bacterial and viral ligands. While we detected hardly any HIV-1 infection in immature vaginal LCs, after stimulation of vaginal LCs with bacterial ligands, HIV-1 infection strongly increased . Thus, our study emphasizes the adverse role inflammation and bacterial infection plays in HIV-1 infection and dissemination.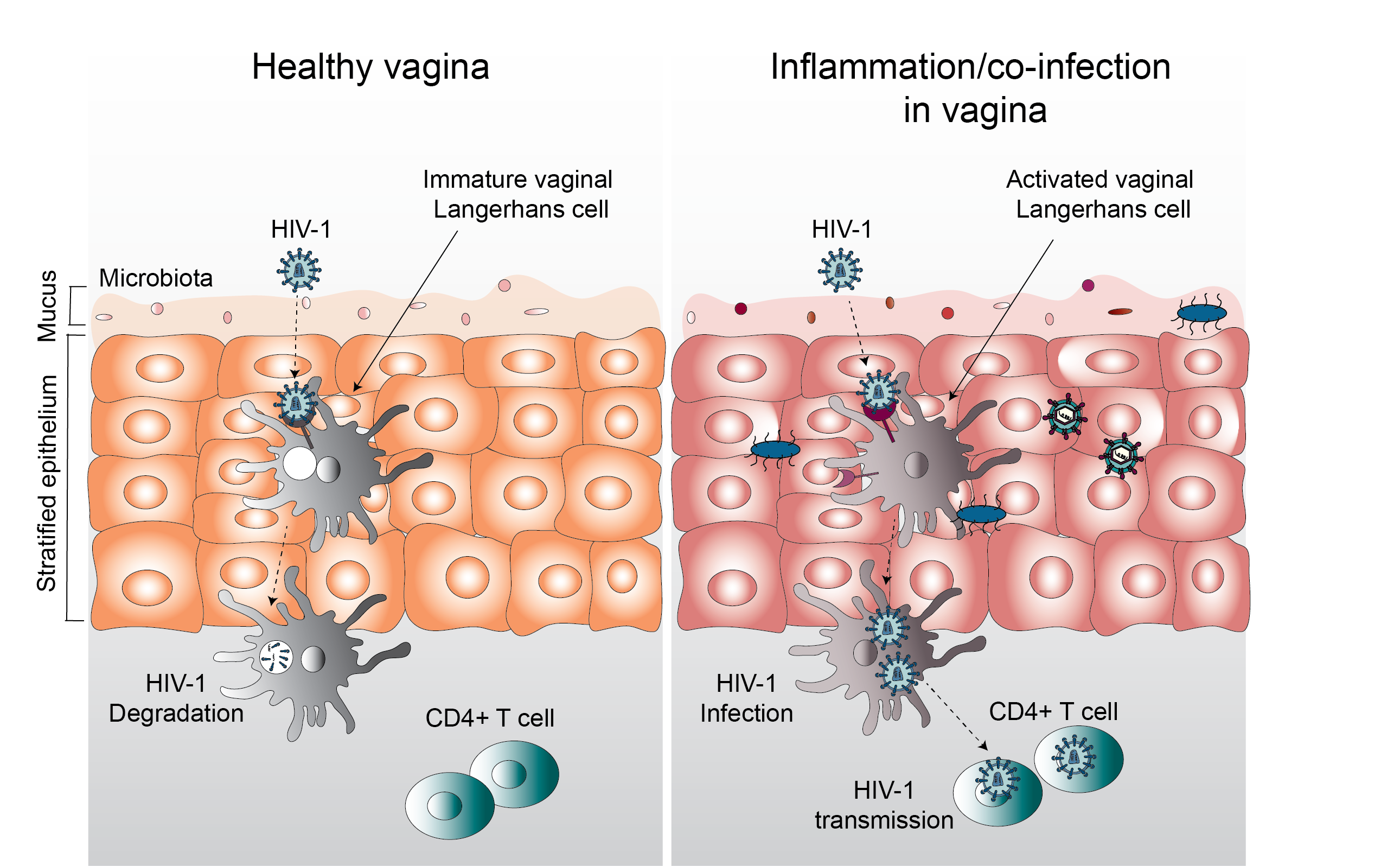
In summary, using a human vaginal explant model, we showed that HIV-1 readily engages with vaginal LCs and that the activation state of LCs is paramount for subsequent infection outcome. Moreover, we could show that vaginal LCs are not identical to those found in the skin even though expression of both CD1a and langerin are high in both cell types. The expression of a wide range of TLRs on vaginal LCs and their ability to become activated by bacterial stimuli indicates that the inflammatory environment of the vaginal mucosa and microbiota play an important role in HIV-1 susceptibility and risk of infection. Studying HIV-1 infection of human cells directly stationed at virus entry sites enables investigations into early host-defense mechanisms against HIV-1 without the need for animal models. The possibility to treat the tissues and stimulate cells inside those tissues further provides opportunities to study viral therapies and preventative methods against HIV-1.
Follow the Topic
-
Scientific Reports

An open access journal publishing original research from across all areas of the natural sciences, psychology, medicine and engineering.
Related Collections
With Collections, you can get published faster and increase your visibility.
Reproductive Health
Publishing Model: Hybrid
Deadline: Mar 30, 2026
Women’s Health
Publishing Model: Open Access
Deadline: Feb 28, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in