Skeletal muscle and aging
In humans, the more than 600 skeletal muscles represent around 40% of our body mass 1. They are responsible for our volunteer movements and are also important for our posture. However, over-time, skeletal muscle aging, named sarcopenia, triggers 30 to 50% of skeletal muscle loss and a decline on muscle strength. It encompasses a decrease in physical activity and might be an obstacle for accomplishing daily life tasks. Sarcopenia represents an increase of frailty and co-morbidity risk. Surprisingly, loss of skeletal muscle mass starts approximately at 40 years old, long before the major aging symptoms 2.
Apart from the depletion of satellite progenitor cells, the mechanisms behind of skeletal muscle cell (myofiber) aging are still elusive. Some aging contributors, such as telomere homeostasis, have been disregarded due to myofiber post-mitotic status.
Telomeres, a hallmark of aging
Telomeres are complex structures located at the end of eukaryote chromosomes3. Their critical function is to distinguish chromosomes ends from double-strand breaks and prevent chromosome fusions. Thus, telomeres guarantee genome stability and it disturbance has been considered as a hallmark of aging 4. Telomeric DNA length decreases progressively during each round of cell division. Critical short telomeres lead to cell cycle arrest and replicative senescence 5.
Telomeres are composed by DNA repeats and a capping protein complex, known as the shelterin complex, a non-coding RNA (TERRA) and a set of factors having functions in DNA repair and of replication 3. In humans, among the telomeric proteins, there is the Telomere Repeat binding Factor 2, TRF2, which hides chromosome extremities from the DNA damage repair machinery and is involved in several aging related pathways (e.g., genome stability, senescence, immune system regulation and mitochondria homeostasis) 6–11.
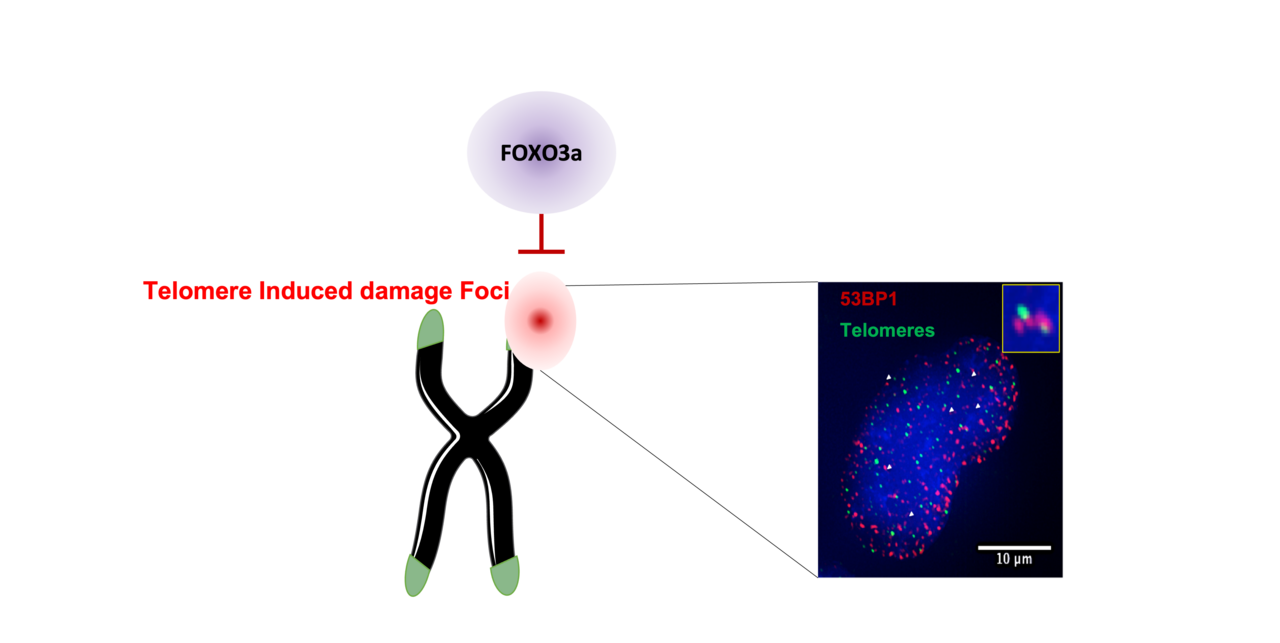
In dividing cells, TRF2 downregulation triggers telomeric DNA damage induced foci or TIFs, cell cycle arrest and senescence. Strikingly, in skeletal muscle fibers, the absence of TRF2 does not lead to detectable telomere damage, suggesting that an alternative telomere protective mechanism exists in these cells 10. In contrast, mitochondrial dysfunction and increased reactive oxygen species (ROS) production were detected. Consequently, the transcription factor FOXO3a was translocated into the nucleus. Interestingly, among the shelterin proteins, only TRF2 expression decreases during aging in human skeletal muscle biopsies. This downregulation was already detected in young adults in their 20s10.
What did we discover?
ROS accumulation might activate signalling pathways protecting telomeres. Here, we treated human myotubes downregulated for TRF2 with antioxidants, then, we noticed an increase of specific telomeric DNA damage or sTIFs. Hence, we thought that one or several factors activated by ROS increase should be a telomeric protective factor. As we previously observed that FOXO3a is translocated into the nuclei of myotubes with decreased TRF2 expression, we tested it as a candidate for telomere protection.
FOXO3a (Forkhead box O3), is a member of the FOX transcription factors. The subclass O: FOXO, is conserved among metazoans 12. The canonic role of FOXO3a, as a transcription factor, is to activate or suppress gene transcription by binding to promoter regions, helped by co-activators 13. Actually, FOXO3a regulates gene expression involved in a numerous of cellular pathways such as oxidative stress response, autophagy, apoptosis, stem cell maintenance, etc. It is considered as a stress response factor and it is associated to homeostasis 14. Strikingly, FOXO3a appears as a central major regulator to ensure survival and it is activated by oxidative stress. Thus, it was a perfect candidate for skeletal muscle telomere protection.
Supporting this hypothesis, our results showed that, FOXO3a protects telomeres in myotubes with TRF2 downregulation, as sTIFs increased when both TRF2 and FOXO3a where downregulated. Moreover, these damages were recognized by the ATM related DNA damage repair pathway. Interestingly, we found that FOXO3a associates to telomeres in myotubes downregulated for TRF2 and in skeletal muscle biopsies at different ages (foetal, young adult, elder). Counterintuitively, the less TRF2 there is in human muscle, the most FOXO3a binds to telomeres.
Next, we showed the specific telomere protection mechanism and the association with TRF2 were conserved in transformed human foreskin fibroblasts, BJ-HELT cells, downregulated for TRF2.
Telomeric DNA damage increases during aging and it is also induced by different external stressors. In order to test whether FOXO3a protects telomeres from genotoxic stress, BJ-HELT cells were exposed to bleomycin, UV or to starvation. Among them, FOXO3a specifically protected against bleomycin induced telomeric damage. Bleomycin induces DNA damage recognized by the ATM pathway. As expected, starvation triggered FOXO3a nuclear translocation but, interestingly, FOXO3a failed to interact with telomeres in this context. Therefore, our results suggest, FOXO3a is differently regulated in conditions known to activate this transcription factor canonical-function than during telomeric stress.
Next, we looked forward to determine which domain of FOXO3a is involved in the telomere protection function. FOXO3a is divided in five main domains, including three conserved regions, two transactivation domains and Forkhead domain or DNA binding domain, which is required for the transcription factor role. Using domain-lacking mutants transduced and downregulating the endogenous FOXO3a in BJ-HELT cells, we observed that the conserved region 2 (CR2) is required for telomere protection upon bleomycin treatment. Finally, we used the same mutant overexpressing vectors in human myotubes downregulated for TRF2 and for the endogenous FOXO3a. Again, our results suggest CR2 is also responsible for the telomere homeostasis in human myotubes, when TRF2 expression is decreased.
Overall, we discovered a direct link between two major longevity hallmarks, the survival factor FOXO3a 15 and telomere protection 16–18. Our results suggest that telomeres are protected in a favoured manner by FOXO3a. This connection is likely involved in muscle aging and it will be of major interest to characterise this link over life time.
References
- Chal, J. & Pourquié, O. Making muscle: skeletal myogenesis in vivo and in vitro. Development 144, 2104–2122 (2017).
- McCormick, R. & Vasilaki, A. Age-related changes in skeletal muscle: changes to life-style as a therapy. Biogerontology 19, 519–536 (2018).
- Giraud-Panis, M.-J., Pisano, S., Poulet, A., Le Du, M.-H. & Gilson, E. Structural identity of telomeric complexes. FEBS Letters 584, (2010).
- López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. Hallmarks of aging: An expanding universe. Cell 186, 243–278 (2023).
- Gilson, E. & Géli, V. How telomeres are replicated. Nat Rev Mol Cell Biol 8, 825–838 (2007).
- de Lange, T. Shelterin-Mediated Telomere Protection. Annu Rev Genet 52, 223–247 (2018).
- Abdallah, P. et al. A two-step model for senescence triggered by a single critically short telomere. Nat Cell Biol 11, 988–993 (2009).
- Sahin, E. et al. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature 470, 359–365 (2011).
- Barnes, R. P., Fouquerel, E. & Opresko, P. L. The impact of oxidative DNA damage and stress on telomere homeostasis. Mech Ageing Dev 177, 37–45 (2019).
- Robin, J. D. et al. Mitochondrial function in skeletal myofibers is controlled by a TRF2-SIRT3 axis over lifetime. Aging Cell 19, e13097 (2020).
- Ye, J. et al. TRF2 and apollo cooperate with topoisomerase 2alpha to protect human telomeres from replicative damage. Cell 142, 230–242 (2010).
- Bridge, D. et al. FoxO and Stress Responses in the Cnidarian Hydra vulgaris. PLOS ONE 5, e11686 (2010).
- Webb, A. E., Kundaje, A. & Brunet, A. Characterization of the direct targets of FOXO transcription factors throughout evolution. Aging Cell 15, 673–685 (2016).
- Salih, D. A. & Brunet, A. FoxO transcription factors in the maintenance of cellular homeostasis during aging. Current Opinion in Cell Biology 20, 126–136 (2008).
- Martins, R., Lithgow, G. J. & Link, W. Long live FOXO: unraveling the role of FOXO proteins in aging and longevity. Aging Cell 15, 196–207 (2016).
- Foley, N. M. et al. Growing old, yet staying young: The role of telomeres in bats’ exceptional longevity. Science Advances 4, eaao0926 (2018).
- Wirthlin, M. et al. Parrot Genomes and the Evolution of Heightened Longevity and Cognition. Curr Biol 28, 4001-4008.e7 (2018).
- Augereau, A. et al. Naked mole rat TRF1 safeguards glycolytic capacity and telomere replication under low oxygen. Sci Adv 7, eabe0174 (2021).
Follow the Topic
-
Communications Biology

An open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of the biological sciences, representing significant advances and bringing new biological insight to a specialized area of research.
Related Collections
With Collections, you can get published faster and increase your visibility.
Signalling Pathways of Innate Immunity
Publishing Model: Hybrid
Deadline: Feb 28, 2026
Forces in Cell Biology
Publishing Model: Open Access
Deadline: Apr 30, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in