Thermal Stability of LiMn2O4 Cathodes in Solid-State Battery Interfaces
Solid-state batteries (SSbs) are considered the frontier of energy storage innovation. By replacing flammable liquid electrolytes with solid alternatives, they promise safer operation, higher energy density, and extended cycle life. While, the transition from laboratory prototypes to scalable devices is hindered by challenges at the electrode-electrolyte interface. Stability at this junction governs ionic transport, structural integrity, and overall device performance.
Oxide-salt solid electrolytes have emerged as attractive candidates due to their chemical robustness, ambient stability, and wide electrochemical windows. Unlike sulfide-based electrolytes, which are highly hygroscopic and degrade rapidly in air, oxide-salt systems remain stable under ambient conditions and are compatible with lithium metal and high-voltage cathodes. However, their integration often requires high-temperature processing (e.g., melt-quenching above 500°C), which risks destabilizing electrode materials.
Our recent study [1] directly addressed this issue by examining the thermal stability of LiMn2O4 cathodes coated with oxide-salt electrolytes. Through a combination of structural, thermal, and electrochemical analyses, we identified critical temperature thresholds beyond which irreversible degradation occurs (Figure 1).
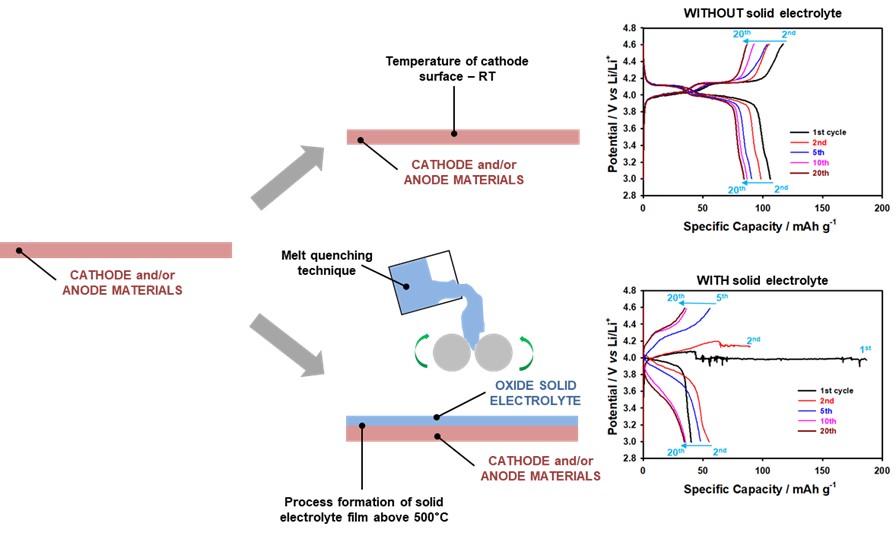
Figure 1. Structural and electrochemical comparison of LiMn2O4 cathodes with and without oxide solid electrolyte integration.
Background: Why LiMn2O4?
LiMn2O4 is a spinel-structured cathode material valued for its low cost, environmental benignity, and relatively high operating voltage (~4 V vs. Li/Li+). Its three-dimensional framework supports fast lithium-ion diffusion, making it suitable for high-power applications. However, LiMn2O4 is susceptible to Jahn–Teller distortion and manganese dissolution, particularly under elevated temperatures or high-voltage cycling. These vulnerabilities make it an ideal model system for probing thermal stability during electrolyte integration.
Experimental Approach
LiMn2O4 was synthesized via solid-state reaction from Li2CO3 and MnO2 precursors, followed by heat treatments at 400°C and 750°C. The oxide-salt electrolyte employed was a ternary glass of Li2O– Li2SO4–B2O3 (60–20–20 mol%) having high ionic and low electronic conductivities are 3.1·10−6 S cm−1 and 2.4·10−12 S cm−1, prepared by melt-quenching. Electrolyte films (~150 µm thick) were applied to cathodes at processing temperatures of 60°C, 250°C, and 500°C.
Characterization techniques included:
- X-ray diffraction (XRD): phase identification and structural evolution.
- Scanning electron microscopy (SEM/EDS): morphology and elemental distribution.
- Thermogravimetric/differential thermal analysis (TGA/DTA): decomposition and oxygen release.
- Electrochemical methods (CV, galvanostatic cycling, EIS): capacity, polarization, and resistance evaluation.
Results
Structural Integrity
- Pristine LiMn2O4 exhibited a well-defined spinel structure.
- At 250°C, partial oxidation of graphite additives occurred, but the spinel lattice remained intact.
- At 500°C, severe degradation was observed, including Mn2O3 formation, current collector oxidation, and collapse of the spinel framework.
Thermal Behavior
TGA revealed three stages of weight loss:
- <350°C: removal of absorbed water.
- 350–500°C: decomposition and oxygen evolution, associated with MnO2/Mn2O3
- 500°C: structural breakdown and side reactions.
Mixtures of LiMn2O4 with solid electrolyte exhibited complex exo-/endothermic peaks, indicating unstable interactions at elevated temperatures. Thermodynamic probability of the reactions calculated using equation: ΔGo298 = Σ ΔGo298 (products) – Σ ΔGo298 (reactants) (Eq. 1), where Isobaric-isothermal potential of the reaction, ΔGo298, kJ mol–1:
(1) 2Fe (substrate) + O2 (air) → 2FeO (-502 kJ mol–1)
(2) 4LiMn2O4 + C (graphite) → 4LiMnO2 + 2Mn2O3 + CO2↑ (-6535 kJ mol–1)
(3) 2LiMn2O4 + Fe (substrate) → 2LiMnO2 + Mn2O3 + FeO (-2373 kJ mol–1)
Electrochemical Performance
- Pristine cathodes delivered high specific capacity, low polarization, and stable cycling.
- Electrolyte-coated cathodes processed at 250–500°C showed reduced current and capacity, lower discharge performance, and higher polarization.
- Impedance spectroscopy confirmed increased resistance (196 Ohm vs. 112 Ohm for pristine cathodes).
Degradation mechanisms included current collector oxidation, partial destruction of the spinel lattice, and structural distortion during lithium insertion/extraction.
Critical Thresholds
Our study identified 300°C as a critical upper limit for processing LiMn2O4 cathodes with oxide-salt electrolytes. Beyond this threshold, irreversible structural and electrochemical degradation occurs. This finding has direct implications for manufacturing: high-temperature melt-quenching is unsuitable for integrating oxide-salt electrolytes with spinel cathodes. Instead, low-temperature deposition techniques (e.g., sol–gel, sputtering, solution-based methods) should be prioritized.
Broader Implications
This work highlights a fundamental principle in battery design: materials must be processed within their stability windows. Just as minerals form under specific geochemical conditions, cathode materials retain their functionality only within defined thermal limits. Exceeding these limits transforms the material into less useful phases.
For solid-state batteries, interface engineering must therefore respect both chemical compatibility and thermal boundaries. Developing scalable, low-temperature methods for electrolyte integration is essential to preserve cathode integrity while ensuring robust ionic conduction.
Connection to the Wider Field
Our findings align with broader research emphasizing interfacial stability:
- Tarascon & Armand [2] underscored stability challenges in rechargeable lithium batteries.
- Goodenough & Park [3] highlighted the balance between electrode performance and electrolyte compatibility.
- Manthiram [4] emphasized interface engineering in solid-state systems.
- Janek & Zeier [5] reviewed the integration challenges of solid electrolytes.
- In our work [1], these studies form a coherent narrative: mastering the interface is central to advancing solid-state batteries.
Conclusion
LiMn2O4 cathodes lose structural and electrochemical stability when subjected to electrolyte film formation above 300°C. Degradation arises from phase decomposition, current collector oxidation, and lattice distortion, leading to reduced capacity and increased resistance. These results establish clear processing limits and highlight the need for innovative low-temperature strategies to realize the potential of oxide-salt solid electrolytes in solid-state batteries.
Ultimately, the research illustrates that nature sets boundaries. Just as minerals transform under volcanic heat, cathodes transform under excessive processing temperatures. Respecting these boundaries is not a constraint but a pathway to designing safer, more reliable, and sustainable energy storage systems.
Author’s Perspective
As one of the authors, my motivation was to bridge fundamental electrochemistry with practical engineering. Having worked across Europe and Asia, I have seen how battery innovation depends not only on novel materials but also on respecting the subtle rules of stability and processing. This project demonstrated that even promising solid electrolytes can undermine cathode performance if thermal limits are ignored.
The broader lesson is that scientific rigor must meet practical design. By combining careful characterization with clear communication, we can guide the community toward safer, scalable solutions. Solid-state batteries are not merely a technological challenge, they are part of a global effort to build sustainable energy systems. I hope this work inspires further exploration of low-temperature processing routes and interface engineering strategies that align with both chemistry and nature’s boundaries.
For further details about the thermal stability between LiMn2O4 cathode and glassy solid electrolytes developed by researchers at the Ukrainian State University of Chemical Technology, we invite you to read our recent publication in the Journal of the Korean Ceramic Society: “Thermal stability of active electrode material in contact with solid electrolyte” https://link.springer.com/article/10.1007/s43207-021-00164-y.
References
- Tron, A., Nosenko, A., Mun, J. Thermal stability of active electrode material in contact with solid electrolyte. Journal of the Korean Ceramic Society 59, 193–201 (2022).
- Tarascon, J.-M., Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 414, 359–367 (2001).
- Goodenough, J. B.,Park, K.-S. The Li-ion rechargeable battery: a perspective. J. Am. Chem. Soc. 135, 4, 1167–1176 (2013).
- Manthiram, A. A reflection on lithium-ion battery cathode chemistry. Nat. Commun. 11, 1550 (2020).
- Janek, J., Zeier, W. G. A solid future for battery development. Nat. Energy 1, 16141 (2016).
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in