Three-dimensional covariance-map imaging of molecular structure and dynamics on the ultrafast timescale
Published in Chemistry
The experiments reported in the paper build on around ten years or so of work in the general area of chemical reaction dynamics. The scattering - or velocity - distribution of a reaction product (see below for examples) reflects the forces acting during the reaction, providing a unique signature for each chemical process and direct insight into the reaction mechanism. Since the late 1980s, ion imaging [1], and later velocity-map imaging [2], have enabled the complete scattering distribution for a chosen reaction product to be measured in a single experiment.

For a long time, VMI was limited to recording images for one reaction product per experiment. Over the past ten years or so we have been developing the technique to enable it to be applied to large chemical systems in which there are usually multiple competing reaction channels and many different products that must be imaged. Our ultra-fast camera, known as the PImMS (or Pixel Imaging Mass Spectrometry) camera (see http://pimms.chem.ox.ac.uk for details), coupled with a range of ‘universal’ ionisation methods (reaction products must be ionised in order to be detected), now allow us to ionise and image every reaction product within a single measurement.
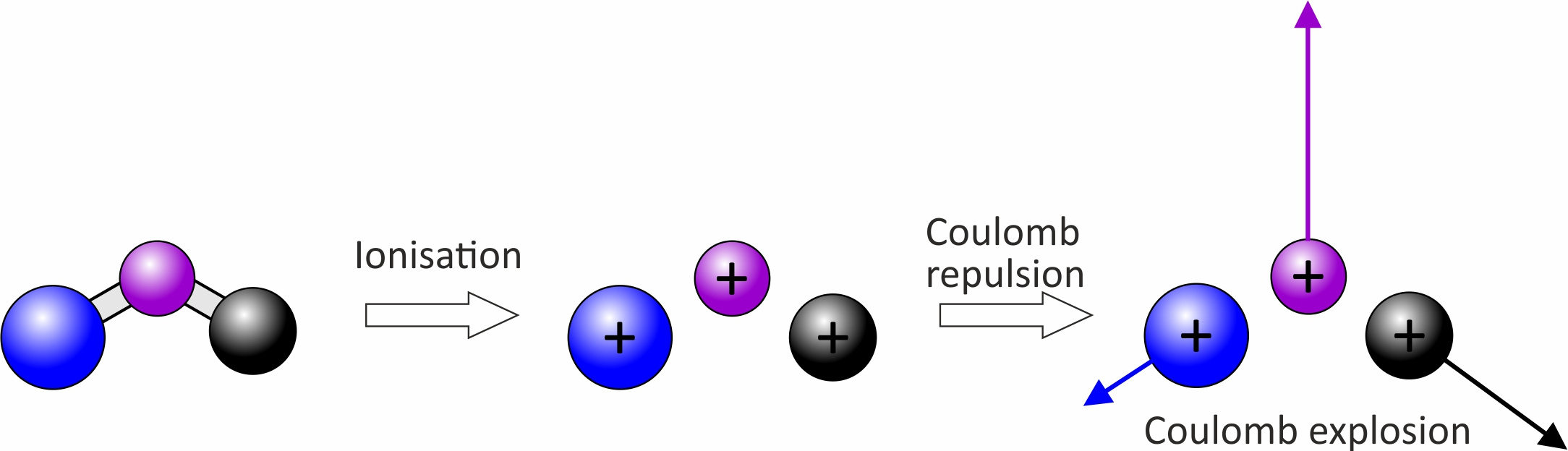
Around five years ago we discovered that we could use our new technology to record images for the products of Coulomb explosions [3,4]. A Coulomb explosion is initiated when a large number of valence electrons are stripped almost instantaneously from a molecule, usually by a short (tens of femtoseconds) intense laser pulse. In extreme cases this leaves behind a collection of atomic ions, whose mutual repulsion causes the molecule to ‘explode’. During the explosion the initial positions of the atoms are mapped onto their final velocities, with the result that by measuring the final velocities by VMI, we can in principle reconstruct the initial molecular structure. Coulomb explosions coupled with VMI offer a new type of universal detection method, providing a snapshot of molecular structures on the femtosecond timescale of the laser pulse. We have demonstrated this approach previously in two dimensions, but the work reported in the present manuscript is the first time we have been able to perform these measurements directly in three dimensions.
The experiments were performed using the Artemis laser at the UK’s Central Laser Facility. After an initial disastrous allocation of beam time, which was terminated by a combination of laser malfunctions, a site-wide power cut, and even a small fire, we fared rather better during a rescheduled attempt. We were able to record both two-dimensional and three-dimensional data sets for a number of small molecules, with the present manuscript summarising our results for Coulomb explosions of CF3I. Our hope is that these first proof-of-concept results will pave the way for further development of the approach into a general-purpose ultrafast method for probing chemical structure and reactivity.
- D. W. Chandler and P. L. Houston, J. Chem. Phys., 87, 1445 (1987).
- A. T. J. B. Eppink and D. H. Parker, Rev. Sci. Instrum., 68, 3477 (1997).
- C. S. Slater, S. Blake, M. Brouard, A. Lauer, C. Vallance. J. J. John, R. Turchetta, A. Nomerotski, L. Christensen, J. H. Nielsen, M. P. Johansson, and H. Stapelfeldt, Phys. Rev. A., 89 011401(R) (2014).
- C. S. Slater, S. Blake, M. Brouard, A. Lauer, C. Vallance, C. S. Bohun, L. Christensen, J. H. Nielsen, M. P. Johansson, and H. Stapelfeldt, Phys. Rev. A., 91, 053424 (2015).
Follow the Topic
-
Communications Chemistry

An open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of the chemical sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
f-block chemistry
Publishing Model: Open Access
Deadline: Feb 28, 2026
Experimental and computational methodology in structural biology
Publishing Model: Open Access
Deadline: Apr 30, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in