Top-down control of human motor thalamic neuronal activity during the auditory oddball task
Published in Neuroscience
The thalamus is a critical brain structure that was considered to act as a relay station between different areas of the brain. It plays a crucial role in regulating sensory perception, motor control, and other cognitive functions. The thalamus receives inputs from various sensory and motor regions of the cortex and sends output to other cortical areas. For many years this concept of a relay function arose from the study of the visual system and was generalized to the rest of the thalamus, but now we realize it interacts with cortex in a more complex manner.
The top-down control of the thalamus refers to the way in which higher-level cortical regions modulate the activity of the thalamus. This modulation can occur through direct or indirect pathways, and it is thought to play a crucial role in cognitive processes such as attention, perception, and decision-making. When the cortex sends top-down signals to the thalamus, it can enhance or suppress the activity of thalamic neurons, depending on the task at hand. For example, when we focus our attention on a particular sensory input, such as a sound or visual cue, the cortex can increase the activity of thalamic neurons that relay information about that stimulus. This increased activity can help to enhance our perception of that stimulus and filter out irrelevant information. This effect can be referred to also as “operant conditioning” of neurons and has been shown in recordings from non-human primates.
Conversely, when we ignore a particular stimulus, the cortex can suppress the activity of thalamic neurons that relay information about that stimulus. This suppression can help to filter out distracting information and maintain our attention on the task at hand. Although sound or visual cues have been studied most frequently, we show in the current study that proprioceptive information ascending through the motor thalamus appears to be controlled in a similar fashion.
In a previous study, we used an auditory oddball task to assess cognitive responses in the globus pallidus internus. The auditory oddball task is a commonly used experimental paradigm that involves presenting a series of repetitive sounds (standard stimuli) interspersed with occasional deviant sounds (deviant stimuli). The participant is asked to silently count the number of deviant tones and report the total at the end of the trial. A correct value confirms the subject attended to the deviant tones. This task is often used to study cognitive processes such as attention, memory, and response inhibition. Several brain regions have been found to be involved in processing the auditory oddball task. The primary auditory cortex, located in the temporal lobe, is responsible for processing sound information, including the detection of deviant stimuli. The superior temporal gyrus is also involved in sensory processing and is believed to play a role in detecting changes in auditory stimuli.
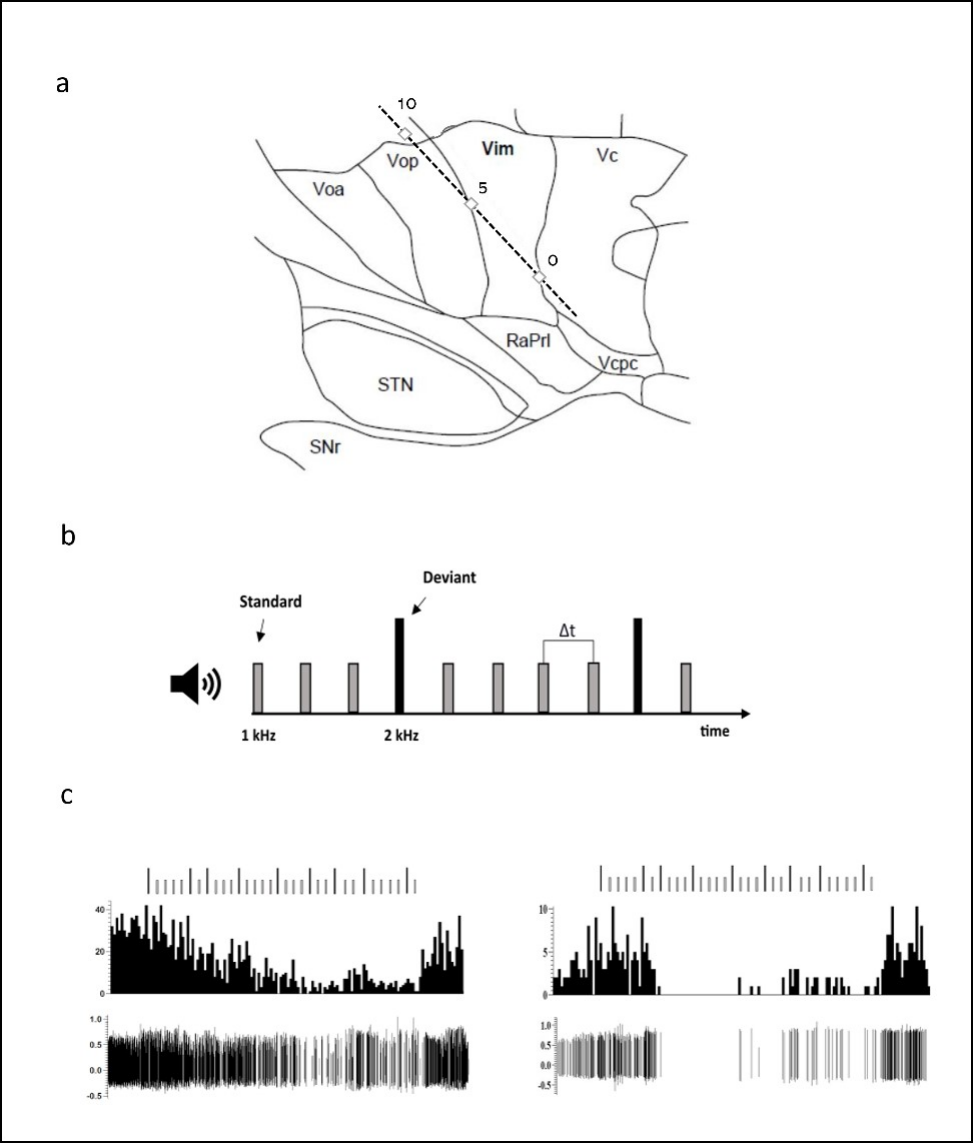
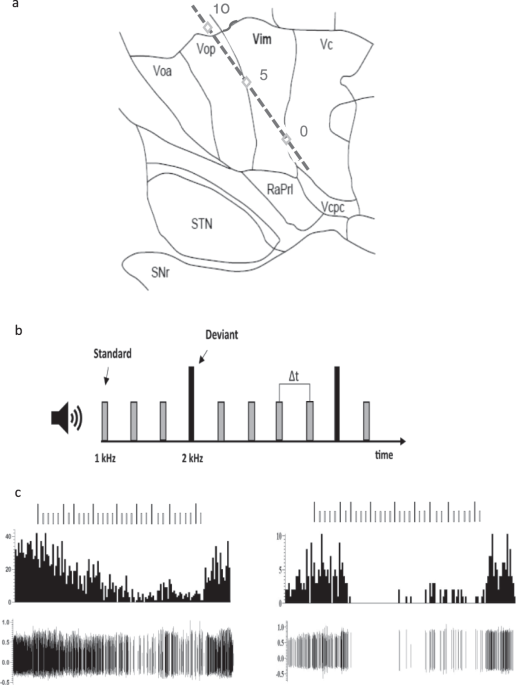
Figure 1. A typical microelectrode track and the behavioral task. A. A representation of the microelectrode track through the motor thalamus targeting the Vim/Vc (0) border passing through Vop. A second series of trajectories (not shown) were more anterior and targeted the Vop/Vim border and passed through Voa. Track is presented on a 14.5mm sagittal map. RaPrl = prelemniscal radiations; Vcpc = ventral caudal parvocellular. B. A schematic representation of the auditory oddball task. C. Examples of two Vim responses to the behavioral task. Vim neuron showed a striking inhibition during the attentional task characterized by a gradual attenuation of the firing (left) or a complete shutdown of the neurons (right).
In addition to these regions, the prefrontal cortex, which is responsible for executive functions such as attention and working memory, is also involved in the processing of the auditory oddball task. The anterior cingulate cortex, which is involved in error detection and response inhibition, has also been found to be active during the task.
Other regions that have been implicated in the processing of the auditory oddball task include the parietal cortex, which is involved in spatial attention and working memory, and the basal ganglia, which are involved in motor control and learning. Indeed, our previous study found many pallidal neurons with a selective response to deviant but not standard tones, in Parkinson’s disease patients undergoing deep brain stimulation implants. We did not see any strong increase in inhibitory output over the course of the task in globus pallidus internus, so we infer that one (or more) of the above-mentioned cortical areas was in top-down control.
In the current study, we investigated the motor thalamus, a downstream structure of the basal ganglia. We found the same selective responses to deviant tones but were initially surprised that some neurons were suppressed or completely inhibited once the task had started, only to bounce back once it was over (Figure 1C). Spiking analysis of histograms showed that motor thalamic neurons responded selectively to the target stimuli by either inhibition or excitation. Indeed, we suggested that these neurons may be the local inhibitory interneurons in the ventral thalamus. Local inhibitory interneurons have been described by Yoland Smith’s group in non-human primates and they receive inputs from cortical areas as well as subcortical areas. These neurons may gate the flow of information from the thalamus to the cortex and may give rise to the responses observed here, instead of an indirect route via the basal ganglia as was initially proposed.
Dysfunction of the top-down control thalamus has been implicated in various neuropsychiatric disorders, such as schizophrenia and attention deficit/hyperactivity disorder (ADHD). In our study, the comparison of PD patients to essential tremor patients revealed much less amplitude modulation of the oscillatory local field potential in the beta range (13-30 Hz) of the response to the deviants in PD patients (Figure 2), who were 12 hours withdrawn from dopamine medication. This suggests that dopamine plays an important role in selective attention and may contribute to the cognitive decline seen in Parkinson’s disease.
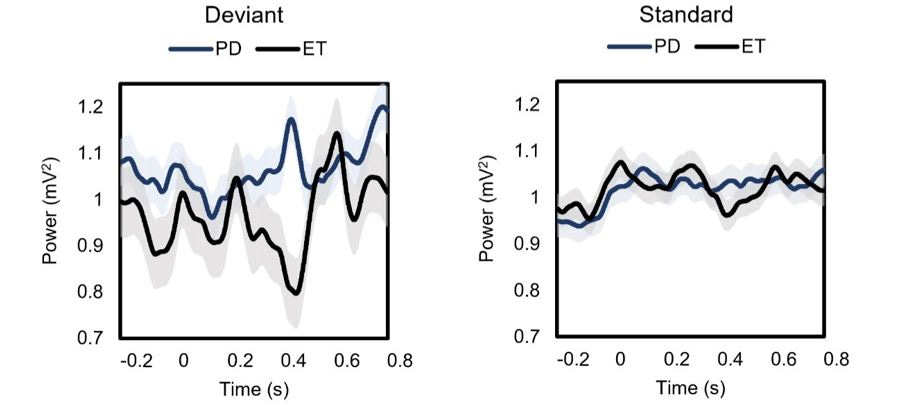
Figure 2. Averages of Beta local field potentials in different disease states. Beta LFPs divided by the disease type. LFPs in the beta band in response to the deviant tones (left) and the standard tones (right). Blue line is Parkinson’s disease tremor (n=6), and black line is essential tremor (n=15). Clear beta modulations (desynchronizations) are observed in the motor thalamus of ET group but not in the PD patients. These comparisons were significant in The Mann-Whitney U test. Results are mean ± SD.
Taken together, this study showed that the ascending proprioceptive information through the motor thalamus seems to be controlled by higher cortical areas as a top-down mechanism. Parkinson's disease can impair this top-down control of selective attention, leading to difficulties in filtering out irrelevant information and maintaining attention. We also showed that beta oscillations may mediate selective attention and that dopaminergic loss in PD may contribute to selective attention deficits. Understanding the neural basis of selective attention in auditory and motor processing may help therapeutic approaches to non motor symptoms in patients with movement disorders.
Follow the Topic
-
npj Parkinson's Disease

This journal publishes original basic science, translational and clinical research related to Parkinson's disease, including anatomy, etiology, genetics, cellular and molecular physiology, neurophysiology, epidemiology and therapeutic development and treatments.
Your space to connect: The Psychedelics Hub
A new Communities’ space to connect, collaborate, and explore research on Psychotherapy, Clinical Psychology, and Neuroscience!
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
Cognition - preclinical models, and preclinical unmet need
Publishing Model: Open Access
Deadline: Jul 27, 2026
Environmental risk factors for Parkinson’s disease
Publishing Model: Open Access
Deadline: May 13, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in