
The quest for batteries with high energy density is a long lasting game with rather simple rules: both cell potential and capacity of the electrode materials need to be enhanced. The “holy grail” in the battery community is to build cells using Li metal anodes, since it combines both high capacity and interesting potential. Yet, such concepts (e.g. Li/air or Li/S) have proven many intrinsic issues related to safety e.g. dendritic growth of Li and mechanistic bottlenecks e.g. polysulfide dissolution or reactivity of radicals to be showstoppers.
In this context the development of rechargeable batteries based on multivalent cations, such as Ca2+ or Mg2+, as charge carriers is promising, yet challenging. The use of Ca or Mg metal anodes could couple the advantages of high theoretical capacity with natural abundance, low cost and safety. Furthermore, the use of divalent charge carriers may account for a two-fold increase in achievable energy density with respect to Li+ for equal amounts of reacted ions. Proof-of-concept was achieved for magnesium with complex electrolyte compositions in 2000 but commercialization is still elusive due to a combination of technological bottlenecks. Most of these issues are being rooted in the poor cation mobility in the cathode material but also in the electrolyte, migration being hampered by strong electrostatic interactions due to the strong polarizing character of Mg2+. We thus thought that an analogous technology based on calcium ion would hold promise for faster reaction kinetics, given the larger size of Ca2+ weaker electrostatic interactions are expected when compared to Mg2+. However, the topic was, at that time, much less explored despite calcium being the fifth most abundant element on earth crust and its standard reduction potential being only 170 mV above that of lithium, thus enabling significantly larger cell potential than the one achievable with magnesium.
Thus, we decided to embark on a risky adventure to assess the viability of calcium metal as battery anodes which should be the first step to develop a battery technology based on calcium. Our first efforts directed to achieve calcium electrodeposition using electrolytes with a wide potential operation window. Despite being disappointed by the first results, we soon understood that ion mobility in the electrolyte may be hindering the process and a moderate increase in temperature unraveled a reversible redox feature in the cyclic voltammogram. At that point, we concentrated in confirming that this feature was indeed related to electrodeposition of calcium, which was somewhat tricky due to air sensitivity of the deposits. (see Figure 1)
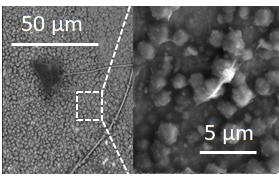
Figure 1. Typical scanning electron micrograph of electrodeposited calcium.
After some effort developing different characterization protocols we were able to unambiguously confirm that the deposit contained calcium metal through synchrotron radiation diffraction (see Figure 2).
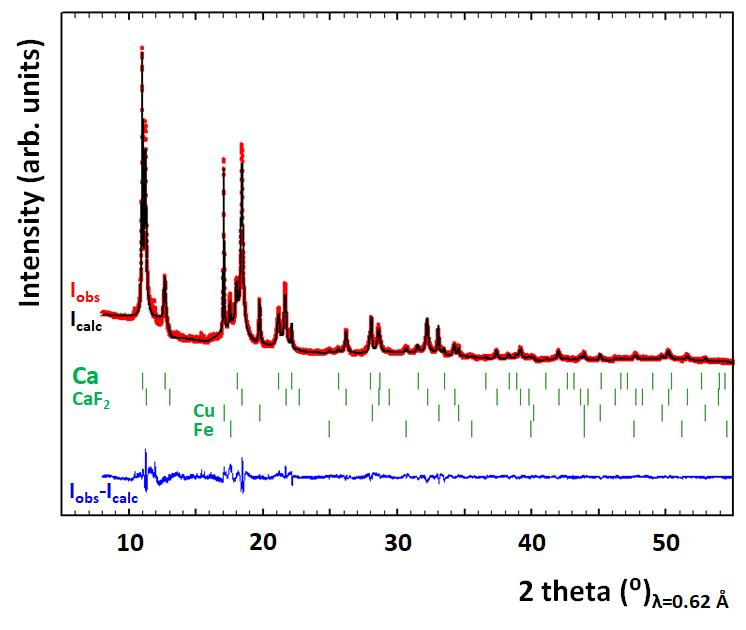
Figure 2. Rietveld refinement (black line) of the synchrotron X-ray diffraction pattern (red circles) corresponding to a calcium deposit after scratching from the substrate and sealing in a borosilicate capillary. Vertical colour ticks denote Bragg positions corresponding to the different phases identified.
The reversibility of the process upon cycling was ascertained and thus these results constituted the first step towards the possible development of high energy density calcium rechargeable batteries. We are currently concentrating in understanding the bottlenecks related to the deposition process: limited coulombic efficiency (related to parasitic electrolyte decomposition) and high polarization. Nonetheless, the first electrolyte generation that we developed already enables further research involving screening cathode materials to achieve proof-of-concept of the technology.
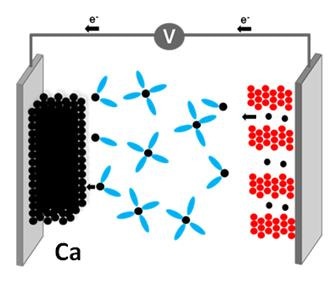
Figure 3: Schematic representation of a battery using a Ca metal anode.
Mastering issues related to migration of divalent ions (both in the solid and in the liquid electrolyte) is currently our main target. If achieved, it should pave the way to technology development. Given the increasing need of batteries not only in terms of amount but also in terms of diversification, research of different and complementary battery technologies to the today prevailing LIBs seems the only long-term sustainable strategy. Herein, and despite all the roadblocks lying certainly ahead, Ca batteries surely have a role to play.
Follow the Topic
-
Nature Materials

A monthly multi-disciplinary journal that brings together cutting-edge research across the entire spectrum of materials science and engineering, including applied and fundamental aspects of the synthesis/processing, structure/composition, properties and performance of materials.



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in