Towards a molecular simulation-driven approach to polymer selection for drug formulation
Published in Chemistry
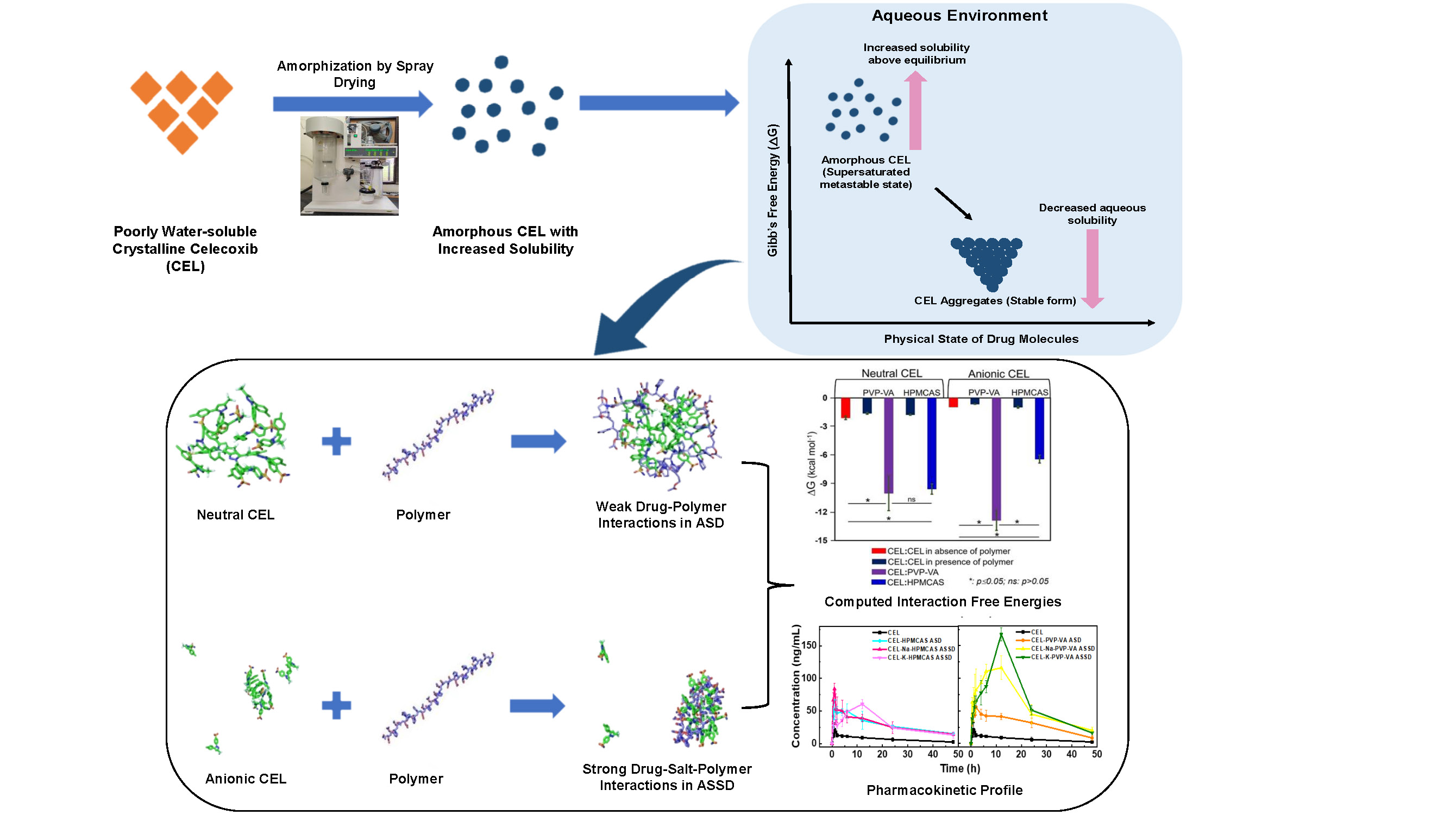
The poorly water-soluble nature of many important drugs poses a challenge for their oral administration and adsorption from the gastrointestinal gut with a high level of bioavailability. Amorphous solid dispersions (ASDs) have emerged as a promising strategy for tackling this problem. In ASDs, the drug is in a high-energy amorphous state and achieves supersaturation exceeding its equilibrium solubility which in turn, enhances its absorption across biological membranes. However, the drug has a propensity to undergo in vivo precipitation in the supersaturated state. Therefore, polymers are often added as excipients to enhance drug solubility and stability in amorphous systems. Selection of a suitable polymer excipient for a given drug has traditionally been guided by trial-and-error or the use of classical empirical physicochemical models to identify thermodynamically miscible systems that, for example, use Hildebrand-Hansen solubility parameters or Flory-Huggins interaction parameters. However, in our study (Mukesh et al., 2023) of celecoxib, a BCS class II poorly water-soluble drug, these methods did not provide correct rankings of the polymers, polyvinylpyrrolidone vinyl acetate (PVP-VA) and hydroxypropyl methylcellulose acetate succinate (HPMCAS), for this purpose.
The simulation of systems of drugs and polymers in atomic detail by molecular dynamics simulation techniques is becoming feasible due to advances in simulation methods and computer hardware. Molecular dynamics simulations provide the possibility of gaining deeper insights into drug-polymer and drug-drug interactions in an aqueous environment, and therefore of providing a better basis for the choice of excipient polymers for optimizing biopharmaceutical performance. In our case study for this approach, we therefore aimed at a systematic comparative experimental and computational analysis of amorphous systems with celecoxib and the two polymers – PVP-VA and HPMCAS. For this purpose, we teamed up to conduct experiments at the National Institute of Pharmaceutical Education and Research (NIPER), S.A.S. Nagar, India, and molecular dynamics simulations at Heidelberg University and the Heidelberg Institute for Theoretical Studies (HITS), Heidelberg, Germany.
Remarkably, the simulations revealed that the drug-polymer intermolecular interactions in aqueous solution were most stable between anionic celecoxib and PVP-VA, correlating well with in vitro and in vivo experimental observations which showed prolonged stability due to strong polar interactions between drug and polymer as revealed by high-end analytical techniques, namely NMR, FTIR and Raman spectroscopy, along with ameliorated solubility, dissolution and pharmacokinetic profile for amorphous salt solid dispersions (ASSD) containing celecoxib and PVP-VA. Drug-drug aggregation was prevented due to stronger drug-polymer interactions for the ionized state of celecoxib in ASSDs compared to the non-ionized state of celecoxib in conventional ASDs. Indeed, the celecoxib-PVP-VA ASSD has the potential for improved biopharmaceutical performance compared to currently marketed formulations of celecoxib, thus enabling lower dosage regimens.
We are currently applying a similar approach to investigate the suitability of polymer excipients for other poorly water-soluble drugs. We anticipate that this approach will be of general value for the generation of supersaturated systems with augmented biopharmaceutical performance, and thus offer the prospect of less frequent administration, lower doses with cost-effectiveness and fewer side effects, thereby increasing patient compliance and improving revenue for pharmaceutical industries.
Follow the Topic
-
Communications Chemistry

An open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of the chemical sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
f-block chemistry
Publishing Model: Open Access
Deadline: Feb 28, 2026
Experimental and computational methodology in structural biology
Publishing Model: Open Access
Deadline: Apr 30, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in