Traces of human specialization in the genome of the yellow fever mosquito
Published in Ecology & Evolution, Microbiology, and Genetics & Genomics

The roots of an unsolved human threat
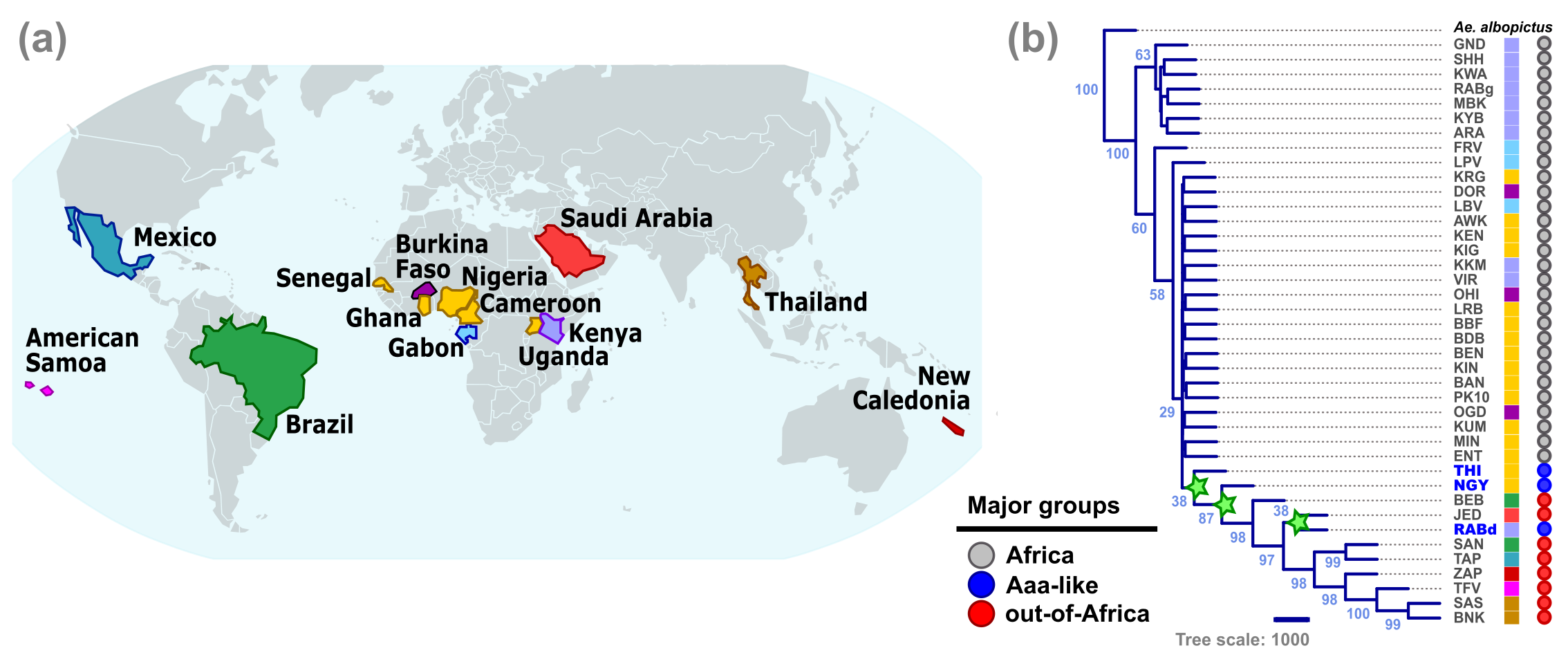
(Re)-Emerging viral diseases worldwide include those caused by viruses transmitted to humans through the bite of arthropods (a.k.a. ‘arboviruses’, short for arthropod-borne viruses), causing more than 1 billion infections and 1 million deaths per year 1. The mosquito Aedes aegypti is the primary global vector of arboviruses such as yellow fever, dengue, chikungunya and Zika viruses 2. Ae. aegypti is also an invasive species that moved out from its native range in Africa to the New World during the transatlantic slave trade 3. This out-of-Africa migration exacerbated the phenotypic differentiation of the two Ae. aegypti ecotypes after their divergence ~5,000 years ago in West Africa 4. The ‘generalist’ Ae. aegypti formosus (Aaf) remained in Africa, whereas the ‘domesticated’ Ae. aegypti aegypti (Aaa) spread globally (Figure 1a).
The Aaa ecotype evolved behaviors of human specialization, such as a preference for biting humans and laying eggs in the clean water of artificial containers, making it also a more efficient vector for transmitting arboviruses than the Aaf ecotype 2,4 (Figure 1b). A deep-rooted hypothesis among vector biologists, dating back to the 1950’s 5, is that the human-specialized behaviors of the Aaa ecotype emerged through self-domestication, whereby species evolve in response to conspecific-exerted selection pressures that mimic domestication, but without the presence of another species (e.g., humans) serving as a ‘domesticator’ 6.
Differentiating Aaa versus Aaf mosquito samples
Our work aimed at identifying genomic signals of adaptation associated with the switch from generalist Aaf to domesticated Aaa behaviors. Such goal is hampered by the complex worldwide population structure of Ae. aegypti 3,4, inconsistent morphological data distinguishing the two ecotypes reliably 3,4,5 (Figure 1a), and the coexistence or genetic admixture of both ecotypes in a few places in Africa and Argentina 3,4. To tackle these challenges, we first identified 314.4 million high-confidence single nucleotide polymorphisms (SNPs) across whole genome sequences (WGS) of 511 African and 123 out-of-Africa mosquitoes from 14 countries (Figure 2a), which were used to perform admixture, population branch statistics, principal component, phylogenetic, and genetic differentiation analyses.
Our results showed that all our samples from out-of-Africa formed a single lineage that endorses a major sub-speciation event between the Aaa and Aaf ecotypes (Figure 2b), as previously reported 3,4. Our findings also show that the sampled out-of-Africa mosquitoes are genetically separated from the African ones, with no evidence of recent genetic admixture events in any of the tested populations. This well-supported correspondence across the geography, population structure, and phylogeny confirmed that the sampled out-of-Africa mosquitoes represent the contemporary Aaa ecotype, allowing the search for genomic traces of its human specialization.
The challenge of detecting ‘variant selection’ within and across Aedes spp.
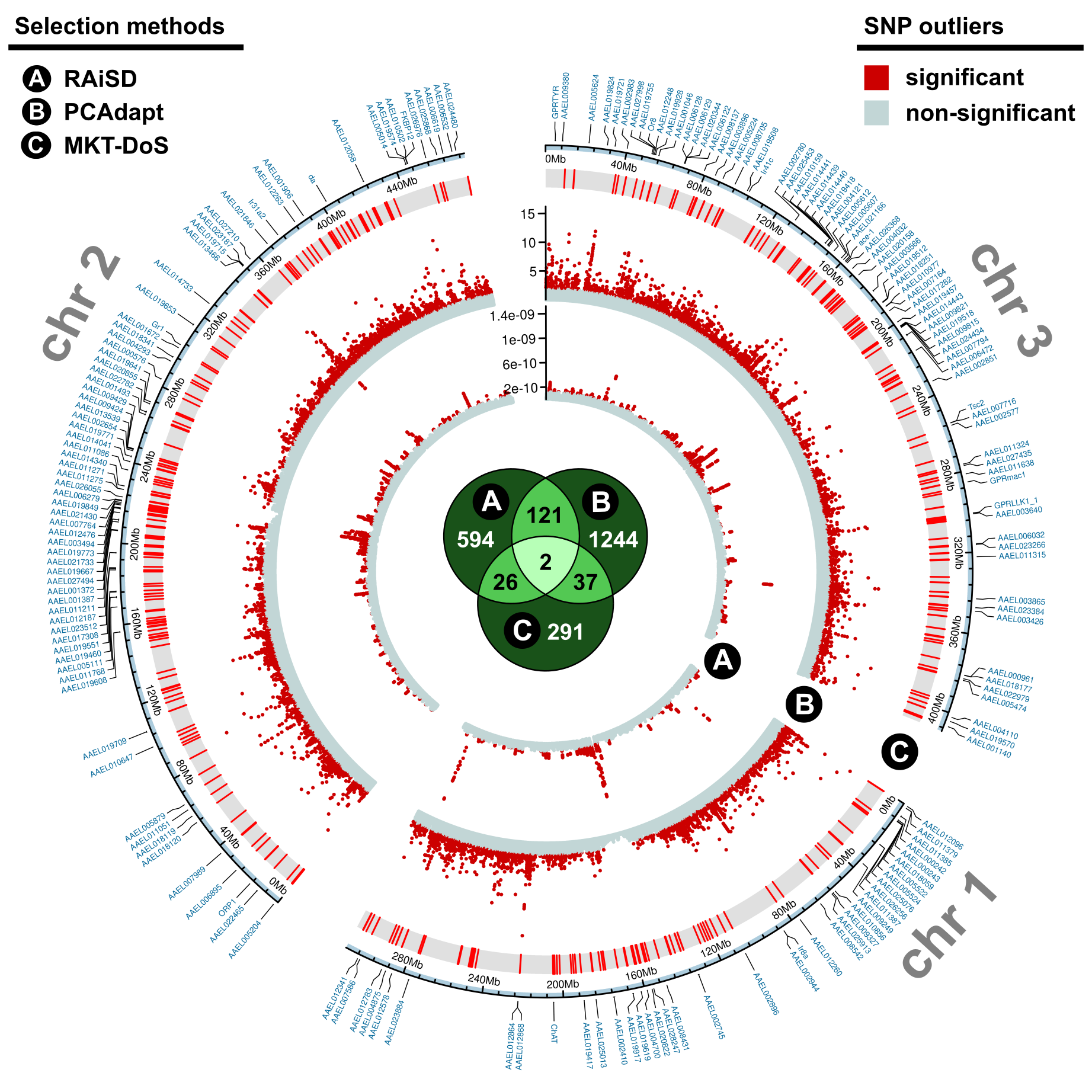
There is increasing evidence of the contribution of ancestral and lineage-specific polymorphisms to sequence divergence and adaptation, particularly for closely related species 7,8. However, it is still unclear whether the methods to identify genomic signals of adaptation within and across species are congruent at different evolutionary time scales 7,8. To tackle this unanticipated challenge, we used three different and complementary methods to identify selection of genetic variants (Figure 3): (a) within hard selective sweeps (RAiSD 9), (b) in variant-outliers concerning population structure (PCAdapt 10), and (c) in protein-coding genes by contrasting polymorphism and divergence data from the closest outgroup Ae. albopictus with the McDonald–Kreitman test (MKT) and its derived direction of selection (DoS) test 11, which reconcile phylogeny-based and population-genetics estimations of gene adaptation 7,8. Each method identified hundreds of candidate adaptive variants at the ‘global’ population scale in out-of-Africa versus African populations and also ‘locally’ for each population. The globally-adaptive variants were used to trace the split between the Aaf and Aaa ecotypes (Figure 3), whereas locally-adaptive variants informed us on the action of selection due to recent environmental and anthropogenic pressures.
Aaa molecular signatures
By intersecting the strongest predictions of global adaptation in out-of-Africa populations from the three methods, we obtained a consensus set of adaptive variants mapping onto one long non-coding RNA and 185 protein-coding genes that we call ‘Aaa molecular signatures’ (Figure 3), which are enriched in chemosensory, neuronal, metabolic and regulatory functions. For instance, several Aaa molecular signature genes encode key ubiquitous chemosensory receptors responsible for intensifying attraction to carbon dioxide and other human-emitted odors, a functional hallmark of self-domesticated behaviors 3,4,5,12. Other Aaa molecular signatures were found in genes related to the reorganization and reallocation of neural resources related to olfaction and sensory compensation 12,13. Interestingly, several Aaa molecular signature genes are located within Quantitative Trait Loci (QTL) previously linked to higher vector competence for Zika virus in mosquitoes from Guadeloupe, Central America (Aaa) versus Gabon, Africa (Aaf) 2. We also found 68 Aaa molecular signature genes with significant allele frequency changes in non-synonymous variants that can accurately differentiate the Aaf from the Aaa ecotype (a.k.a. ‘Aaa markers’) across independent population samples.
Reconciling local adaptation with self-domestication
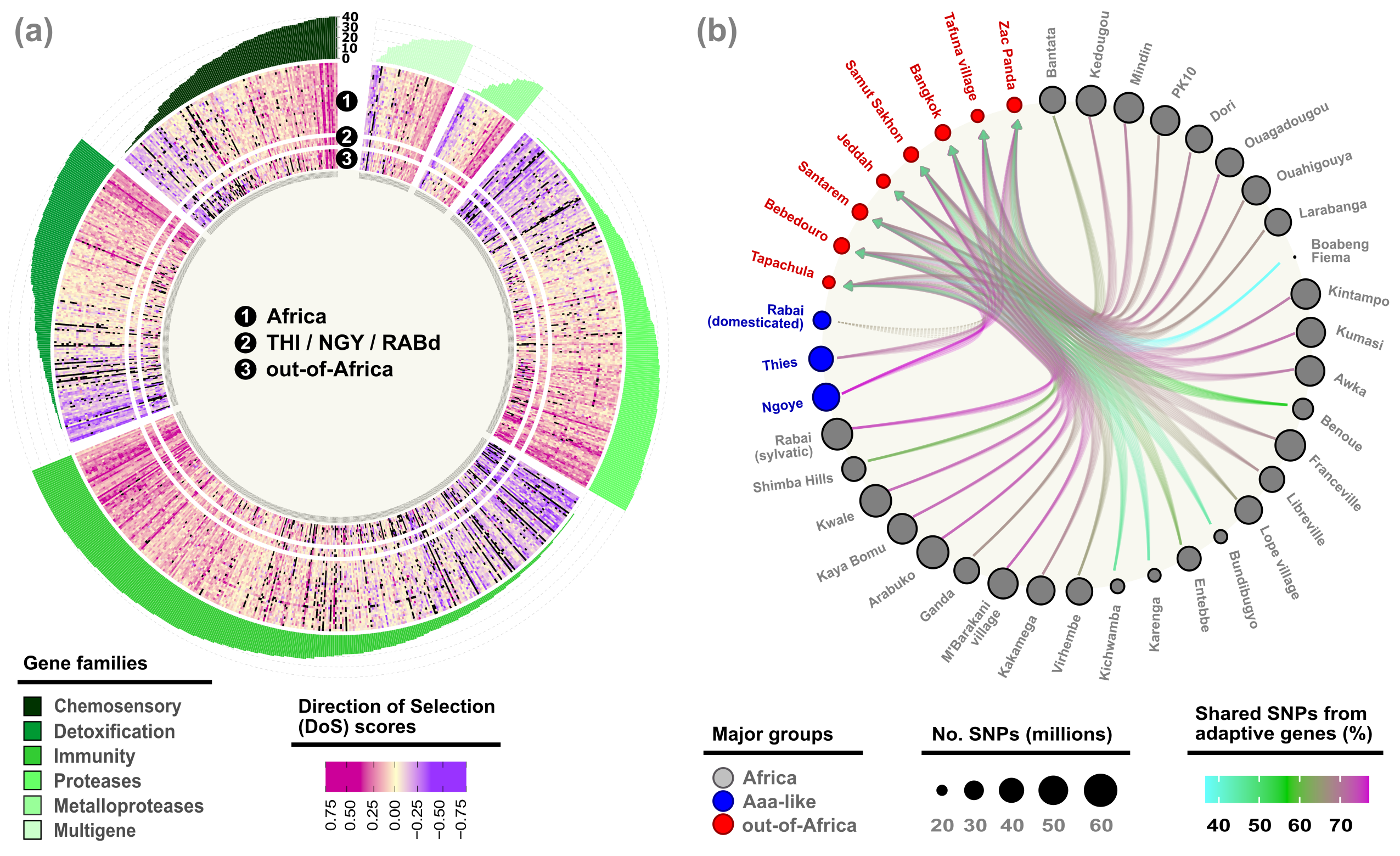
Mosquitoes are sensory specialists that use gustatory (e.g., GR1), odorant (e.g., OR4) and ionotropic (e.g., IR8a) receptors to detect and integrate diverse host-emitted cues 4,12,13. Accordingly, our original hypothesis was to find evidence of a canonical set of olfactory genes supporting the complexity of self-domesticated behaviors in Aaa mosquitoes. However, we were shocked to find extensive local adaptation of protein-coding and non-coding sequences across out-of-Africa populations with a functional redundancy associated with chemosensory, neuronal, detoxification and metabolic activities (Figure 4a), regardless of the selection method and thresholds used to detect them. Two further breakthroughs allowed us to unveil a novel hypothesis for the evolution of self-domestication in Ae. aegypti.
First, a non-canonical odor coding was recently demonstrated in Ae. aegypti 13, whereby the breadth and flexibility of volatile perception is increased by many olfactory neurons co-expressing multiple receptors with different chemical sensitivities, contrasting with the long-standing dogma of one-receptor, one-neuron, one-glomerulus coding of the fruit fly Drosophila melanogaster. Furthermore, we found that hundreds of alleles related to self-domesticated behaviors are evolving neutrally or under weak selection across Ae. aegypti populations (Figure 4a) from both novel and retained ancestral polymorphisms (Figure 4b), suggesting that the neuronal-olfactory redundancy can be recursively explored by mosquitoes.
Altogether, these findings suggest that self-domestication processes have occurred and might continue to occur in Ae. aegypti because rapid ecotype formation is enabled by both the action of selection on preexisting genetic variation and of local adaptation driven by neuronal-olfactory redundancy.
Are genome and organismal complexities coupled in Ae. aegypti?
In comparison to other blood-feeding insects, Ae. aegypti has a very large and highly repetitive genome (>50% of its ∼1,376 million base pairs), which is about 5 and 7 times larger than the genomes of the malaria vector Anopheles gambiae and Dr. melanogaster, respectively 14. Yet, having a larger genome and/or a higher number of genes are not enough (on their own) to boost and do not correlate with organismal complexity, as shown by the “C-G” paradoxes 15,16. Instead, our findings clearly unveil the efficiency of ‘local adaptation’ across out-of-Africa populations to select for gene families with redundant and pleiotropic effects, such as olfaction, detoxification, and neuronal functions (Figure 4a), which are abundantly encoded in the Ae. aegypti genome (i.e., 72 gustatory, 116 odorant and 132 ionotropic genes 12).
Despite a two-fold reduction of polymorphisms in out-of-Africa populations, we also found that Ae. aegypti populations have a rich stock of ancestral, standing and weakly evolving genetic variants (Figure 4b), which can be maintained for longer periods of time beyond neutral expectations 17. Our work and previous studies strongly suggest that standing genetic variation can promote polygenic adaptation of complex phenotypes 17, such as domestication 18 and feralization (i.e., re-adaptation to the wild) 19,6. The further discovery of neuronal-olfactory redundancy in Drosophila and Anopheles species 20 puts forward a pressing research question: Are the evolutionary trends found in Ae. aegypti common (or not) among insects living under anthropogenic environments and with different genome complexities?
Is there a convergent genetic toolkit for the ‘domestication syndrome’?
The recursive redundancy of broad chemosensory, neuronal, hormonal, metabolic and regulatory functions found in our Aaa molecular signatures and out-of-Africa locally adapted genes highlight striking similarities with the functions previously reported for adaptive genes associated with human domestication in rabbits, chickens, cattle, and silkworms 18,21. This finding is in good agreement with growing evidence indicating a repeated evolutionary co-option of genes associated with the fine regulation of neuronal and metabolic functions in both self- and human-driven domestication processes 6,21. We expect that our findings trigger transdisciplinary research on the environmental pressures, genetic traits and physiological mechanisms that might validate a hypothesized ‘domestication syndrome’ (i.e., a suite of shared physical and behavioral domesticated traits) across Bilaterian taxa 6.
Redirecting a study: a worldwide collaborative effort
The origins of this study date back to 2017, with the funding granted to the Bonizzoni laboratory by the Human Frontiers Science Foundation (Grant no. RGP0007), which aimed at testing the hypothesis of adaptive immunity in Ae. aegypti mediated by non-retroviral endogenous viral elements (nrEVEs) 14. To that end, we established the pattern of viral integrations in field samples through extensive sequencing and analyses, and tested for a correlation between viral exposure and viral integrations. We found that the integration of viral sequences in the genomes of Aedes spp. is a rare and continuous event 22,23,24, but it does not correlate with the intensity of viral exposure nor with the shift between the Aaf and Aaa ecotypes.
The inclusion in the team of two postdocs (Alejandro and Irma Lozada-Chávez) with extensive evolutionary biology and bioinformatics expertise helped shift from nrEVEs to whole-genome analyses and search for the genomic basis of ‘self-domesticated’ behaviors in the Aaa ecotype. Interdisciplinarity, dedicated work, High-Performance Computing provided by the Leipzig (Germany) and Pavia (Italy) Universities, and an intense peer-review process, along with an ability to redirect quickly the original grant sources in the pursuit of a bigger goal, were all key for the success of this study.
In addition to having contributed to (as also benefited from) the publicly available wealth of Ae. aegypti sequence datasets, our Aaa markers could be used to unambiguously distinguish the two ecotypes in wild-collected mosquitoes and to design new control strategies for Ae. aegypti populations. This is particularly relevant since recent studies predict a faster and wider worldwide extension and adaptability of Aedes spp. mosquitoes due to climate change 1,22,25.
References:
- Iwamura, T., Guzman-Holst, A. & Murray, K. A. Accelerating invasion potential of disease vector Aedes aegypti under climate change. Nat. Commun. 11, 2130 (2020).
- Aubry, F., Dabo, S., Manet, C., Filipović, I., Rose, N. H., Miot, E. F., ... & Lambrechts, L. Enhanced Zika virus susceptibility of globally invasive Aedes aegypti populations. Science 370, 991–996 (2020).
- Powell, J. R., Gloria-Soria, A. & Kotsakiozi, P. Recent history of Aedes aegypti: vector genomics and epidemiology records. BioScience 68, 854–860 (2018).
- Rose, N. H., Sylla, M., Badolo, A., Lutomiah, J., Ayala, D., Aribodor, O. B., ... & McBride, C. S. Climate and urbanization drive mosquito preference for humans. Curr. Biol. 30, 3570–3579 (2020).
- Tabachnick, W. J., & Powell, J. R. A world-wide survey of genetic variation in the yellow fever mosquito, Aedes aegypti. Genet. Res. 34, 215–229 (1979).
- Hecht, E. E., Barton, S. A., Flattery, C. N. R., & Meza, A. M. The evolutionary neuroscience of domestication. Trends Cogn. Sci. 27, 553–567 (2023).
- Latrille, T., Rodrigue, N. & Lartillot, N. Genes and sites under adaptation at the phylogenetic scale also exhibit adaptation at the population-genetic scale. Proc. Natl. Acad. Sci. USA 120, e2214977120 (2023).
- Vigué, L. & Tenaillon, O. Predicting the effect of mutations to investigate recent events of selection across 60,472 Escherichia coli strains. Proc. Natl. Acad. Sci. USA 120, e2304177120 (2023).
- Alachiotis, N. & Pavlidis, P. RAiSD detects positive selection based on multiple signatures of a selective sweep and SNP vectors. Commun. Biol. 1, 79 (2018).
- Privé, F., Luu, K., Vilhjálmsson, B. J., & Blum, M. G. Performing highly efficient genome scans for local adaptation with R package PCAdapt version 4. Mol. Biol. Evol. 37, 2153–2154 (2020).
- Stoletzki, N. & Eyre-Walker, A. Estimation of the neutrality index. Mol. Biol. Evol. 28, 63–70 (2011).
- Morita, T., Lyn, N. G., von Heynitz, R. K., Goldman, O. V., Sorrells, T. R., DeGennaro, M., ... & Vosshall, L. B. Cross-modal sensory compensation increases mosquito attraction to humans. Sci. Adv.11, eadn5758 (2025).
- Herre, M., Goldman, O. V., Lu, T. C., Caballero-Vidal, G., Qi, Y., Gilbert, Z. N., ... & Younger, M. A. Non-canonical odor coding in the mosquito. Cell 185, 3104–3123 (2022).
- Palatini, U., Contreras, C. A., Gasmi, L., & Bonizzoni, M. Endogenous viral elements in mosquito genomes: current knowledge and outstanding questions. Curr. Opin. Insect Sci. 49, 22-30 (2022).
- Lozada-Chávez, I., Stadler, P. F., & Prohaska, S. J. “Hypothesis for the modern RNA world”: a pervasive non-coding RNA-based genetic regulation is a prerequisite for the emergence of multicellular complexity. Orig. Life Evol. Biosph. 41, 587-607 (2011).
- Alvarez-Ponce, D., & Krishnamurthy, S. Organismal complexity strongly correlates with the number of protein families and domains. Proc. Natl. Acad. Sci. USA 122, e2404332122 (2025).
- Barrett, R. D. H. & Schluter, D. Adaptation from standing genetic variation. Trends Ecol. Evol. 23, 38–44 (2008).
- Xia, Q., Guo, Y., Zhang, Z., Li, D., Xuan, Z., Li, Z., ... & Wang, J. Complete resequencing of 40 genomes reveals domestication events and genes in silkworm (Bombyx). Science 326, 433–436 (2009).
- Andrade, P., Alves, J. M., Pereira, P., Rubin, C. J., Silva, E., Sprehn, C. G., ... & Carneiro, M. Selection against domestication alleles in introduced rabbit populations. Nat. Ecol. Evol. 8, 1543–1555 (2024).
- Task, D., Lin, C. C., Vulpe, A., Afify, A., Ballou, S., Brbic, M., ... & Potter, C. J. Chemoreceptor co-expression in Drosophila melanogaster olfactory neurons. eLife 11, e72599 (2022).
- Andersson, L. & Purugganan, M. Molecular genetic variation of animals and plants under domestication. Proc. Natl. Acad. Sci. USA 119, e2122150119 (2022).
- Crava, C. M., Varghese, F. S., Pischedda, E., Halbach, R., Palatini, U., Marconcini, M., ... & Bonizzoni, M. Population genomics in the arboviral vector Aedes aegypti reveals the genomic architecture and evolution of endogenous viral elements. Mol. Ecol. 30,1594–1611 (2021).
- Marconcini, M., Pischedda, E., Houé, V., Palatini, U., Lozada-Chávez, A. N., Sogliani, D., ... & Bonizzoni, M. Profile of Small RNAs, vDNA Forms and viral integrations in late chikungunya virus infection of Aedes albopictus mosquitoes. Viruses 13, 553 (2021).
- Palatini, U., Alfano, N., Carballar-Lejarazu, R., Chen, X. G., Delatte, H., & Bonizzoni, M. Virome and nrEVEome diversity of Aedes albopictus mosquitoes from La Reunion Island and China. Virology journal 19, 190 (2022).
- Carlassara, M., Khorramnejad, A., Oker, H., Bahrami, R., Lozada‐Chávez, A. N., Mancini, M. V., ... & Bonizzoni, M. Population-specific responses to developmental temperature in the arboviral vector Aedes albopictus: Implications for climate change. Glob. Chang. Biol. 30, e17226 (2024).
Ethics declarations: This an original article written by the Authors, without the use of ChatGPT or any other Artificial Intelligence tool. The Authors declare no competing interests. We thank Cristina Bojórquez Espinosa for proofreading this article.
Poster photo: Female mosquitoes of the generalist Aaf (dark, left) and domestic Aaa (light, right) forms of A. aegypti. Photo credits: Institut Pasteur (2020) / Greg Murray, Blaise Daures and Louis Lambrechts © 2025 CC BY-NC-ND 4.0.
Related Research Briefing article: Lozada-Chávez I. & Bonizzoni M. Genetic adaptations from self-domestication in the yellow fever mosquito. Nat Ecol Evol 9, 545–546 (2025). https://doi.org/10.1038/s41559-025-02649-z
Author affiliations:
- Irma Lozada-Chávez, Postdoctoral Researcher at the Evo-devo, Bioinformatics and Neuromorphic Information Processing groups, Institute of Computer Science and Faculty of Mathematics and Computer Science, Leipzig University, Leipzig, Germany
- Alejandro Nabor Lozada-Chávez, Postdoctoral Researcher at the Department of Biology and Biotechnology, University of Pavia, Pavia, Italy
- Louis Lambrechts, Research Director and Head of the Insect–Virus Interactions Unit, Institut Pasteur, Université Paris Cité, CNRS UMR2000, Paris, France
- Samia Elfekih, Honorary Senior Research Fellow at the Bio21 Institute, School of Biosciences, University of Melbourne, Melbourne, Victoria, Australia
- Mariangela Bonizzoni, Professor at the Department of Biology and Biotechnology, University of Pavia, Pavia, Italy
Cite this article
Lozada-Chavez I., Lozada-Chávez A.N., Lambrechts L., Elfekih S. and Bonizzoni M. Traces of human specialization in the genome of the yellow fever mosquito. Nature Research Communities, April 22 (2025). URL: https://communities.
Follow the Topic
-
Nature Ecology & Evolution

This journal is interested in the full spectrum of ecological and evolutionary biology, encompassing approaches at the molecular, organismal, population, community and ecosystem levels, as well as relevant parts of the social sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Biodiversity and ecosystem functioning of global peatlands
Publishing Model: Hybrid
Deadline: Jul 27, 2026
Understanding species redistributions under global climate change
Publishing Model: Hybrid
Deadline: Jun 30, 2026






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in