
By Alice Zwerling, Sayema Badar, Tassia Araujo, Jeff Pennington & Madhukar Pai
Introdcution: The 2nd Edition of the BCG World Atlas provides user friendly, open access, and easy to access data on the current TB vaccine, and provides the clinician, researcher, and public health practitioner alike with resources and information necessary to interpret current and novel TB diagnostics and conduct fruitful research on novel vaccines.
March 24th is World Tuberculosis (TB) Day. TB affects 10.4 million people each year, and kills 1.8 million. In the past year, we’ve seen TB surpass HIV as the infectious disease responsible for the most deaths globally. While progress has been made in the field of TB research and development, much remains to be done. We’ve seen promising developments in the field of novel drugs and drug regimens for TB. The field of TB diagnostics continues to advance with novel tools and algorithms.
Sadly, no new TB vaccines are around the corner at least for the foreseeable future. Product development partnerships such as Aeras are actively engaged in new vaccine development, with at least 13 vaccine candidates currently being tested clinically. For now, the only vaccine we have for TB is the Bacille Calmette Guerin (BCG) vaccine, first introduced in 1921.
Although the BCG vaccine is used worldwide, mainly to prevent life-threatening TB in infants and young children, it has been ineffective in controlling the global TB epidemic.Despite its variable and modest efficacy, this vaccine continues to be one of the most widely administered vaccines globally and the only vaccine in use for TB.
As we approach a century of BCG use, we must recognize the many controversial elements of this vaccine and the wide variety of policies and practices implemented by countries around the world. As there is no one size fits all solution, countries adopt a diverse range of approaches to suit their needs, and epidemiological trends.
To better understand the diversity of BCG policies and practices and to aid clinicians in choosing the appropriate diagnostic test relative to patients’ BCG status, we launched the World BCG Atlas in 2011 as a web-based resource for clinicians, researchers, and public health staff. Since then, the Atlas has been accessed over 200,000 times and continues to serve as a reference for clinicians, researchers, public health practitioners, and policy makers, providing information on BCG policies and practices from over 180 countries. The Atlas provides information that can aid in both individual clinical decisions ie: the interpretation of TB diagnostics such as tuberculin skin tests, and in defining research questions, including the design of novel TB vaccines.
Since its launch, we have received many requests for updated as well as new data on BCG policies and practices as countries’ policies and practices are constantly changing and being updated. To address these needs, we have launched the second edition of the Atlas. The new edition contains updated data on TB epidemiology, newly collected data on BCG strain type, changes made to and duration of strain type usage, location of BCG vaccine administration, whether tuberculin skin testing (TST) is done pre and post vaccination, specific recommendations for BCG vaccination for HIV-infected infants, BCG manufacturers and suppliers, availability of supply and occurrence of vaccine shortages or stock outs.
The 2nd Edition of the Atlas (home page shown below) includes information on how a country’s BCG policy is updated, and, if available, we have included links to national vaccine policy documents.
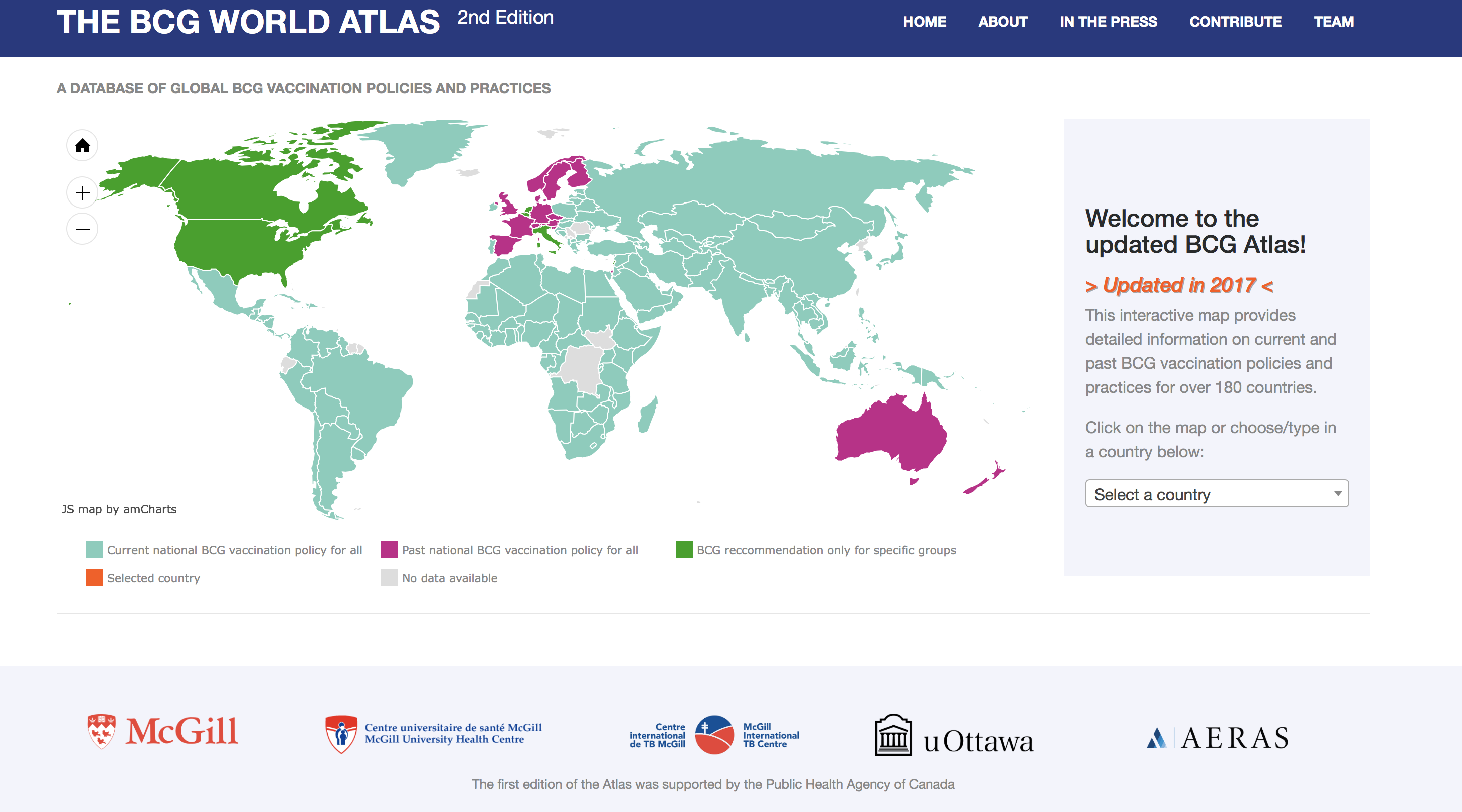
The 2nd Edition of the Atlas shows the continued diversity of BCG policies and practices globally. For example, some countries use tuberculin testing pre-vaccination, to establish eligibility for BCG vaccination, while others use tuberculin testing after vaccination. A small minority of countries recommend tuberculin both pre and post vaccination. These approaches all have important implications on an individual’s BCG status and their likelihood of having a false positive tuberculin test result upon later testing for immigration, pre-employment screening or post-exposure screening.
Another important finding has been the frequent occurrence of vaccine stock-outs, most frequently in 2014, 2015 and continued in 2016. While some countries have changed strain type to cope with lack of availability of the BCG vaccine, others have had to cease BCG vaccination in some cases for years. These findings are a cause for concern, especially for children in whom BCG offers protection, and reinforce the need for a better strategy to ensure the BCG supply chain.
As countries’ BCG policies and practices continue to evolve and change so too must we adapt. The updated Atlas helps both the clinical, public health and research communities by summarizing the diversity of approaches adopted by different countries in a searchable, online, open access database of global BCG vaccination policy.
In the long term, the TB field urgently needs a better vaccine. A vaccine with even 60% efficacy delivered to 20% of adolescents and adults could avert 30 million cases of active TB disease in the first 20 years. So, serious investments and partnerships are required to continue vital R&D efforts, and given the scale of the TB problem, a viable global market exists for new TB vaccines. It is a matter of concern that R&D investments in TB are not keeping up with the need for new tools. According to Treatment Action Group’s 2016 Report on Tuberculosis Research Funding Trends, in 2015, the world spent US$620.6 million on TB R&D the lowest level of funding since 2008.
As we advocate and wait for an improved or new TB vaccine, we hope that resources such as the 2nd Edition of the BCG World Atlas will continue to provide user friendly, open access, and easy to access data on the current TB vaccine, and provide the clinician, researcher, and public health practitioner alike with resources and information necessary to interpret current and novel TB diagnostics and conduct fruitful research on novel vaccines.
Acknowledgements: We are also grateful to Ann Ginsberg and AERAS for their partnership and support. The Public Health Agency of Canada supported the 1st Edition of the BCG World Atlas.
Dr Alice Zwerling, MSc, PhD, is an Assistant Professor (tenure track) of epidemiology at the School of Epidemiology, Public Health & Preventive Medicine, University of Ottawa, Ottawa. Alice received her PhD in Epidemiology from McGill University and completed a postdoctoral fellowship at Johns Hopkins Bloomberg School of Public Health. Her main areas of expertise and interest currently involve cost-effectiveness analyses to guide thoughtful implementation of new tools and treatment regimens for TB. Prior to joining the University of Ottawa, Alice worked with the KNCV Tuberculosis Foundation in The Hague where she continues to provide technical support to national TB programs.
Sayema Badar is a Research Assistant at the McGill International TB Centre, McGill University. She worked as a coordinator of the 2nd edition of the BCG World Atlas.
Tassia Araujo is a student at McGill University, Montreal. She worked as a computer programmer for 2nd edition of the BCG World Atlas.
Jeff Pennington, based in Ottawa, Canada, also provided computer/IT support for the 2nd edition of the BCG World Atlas.
Prof Madhukar Pai, MD, PhD is a Canada Research Chair in Epidemiology & Global Health at McGill University, Montreal. He is the Director of McGill Global Health Programs, and Associate Director of the McGill International TB Centre. He serves as a Consultant to the Bill & Melinda Gates Foundation. He serves on the STAG-TB committee of WHO, Geneva; Scientific Advisory Committee of FIND, Geneva; and Access Advisory Committee of TB Alliance, New York. Twitter: @paimadhu





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in