Transient Shuttle for a Widespread Neural Probe with Minimal Perturbation
Published in Bioengineering & Biotechnology and Materials
In our recent research, we developed an innovative transient shuttle system to address the significant challenge of mechanical mismatch between neural probes and brain tissue. This approach uses a polyvinyl alcohol (PVA) shuttle to implant flexible mesh probes into the brain, ensuring minimal tissue damage and long-term stability.
Long-term monitoring of deep brain activity is crucial for understanding the mechanisms of various neurological diseases. Traditionally, high-rigidity probes are used to penetrate the brain's surface, but the stiffness mismatch between these rigid probes and the soft brain tissue often leads to tissue damage and gliosis, an immune response that degrades probe performance over time. Additionally, brain movements can disturb the stability of recordings when using rigid probes.
Flexible probes offer a promising alternative for chronic brain implants. Their compatibility with the soft brain tissue prevents the degradation of recordings and supports the functional recovery of neurons. However, implanting these flexible probes deep into the brain typically requires a rigid shuttle for assistance, which can cause secondary damage during insertion and withdrawal.
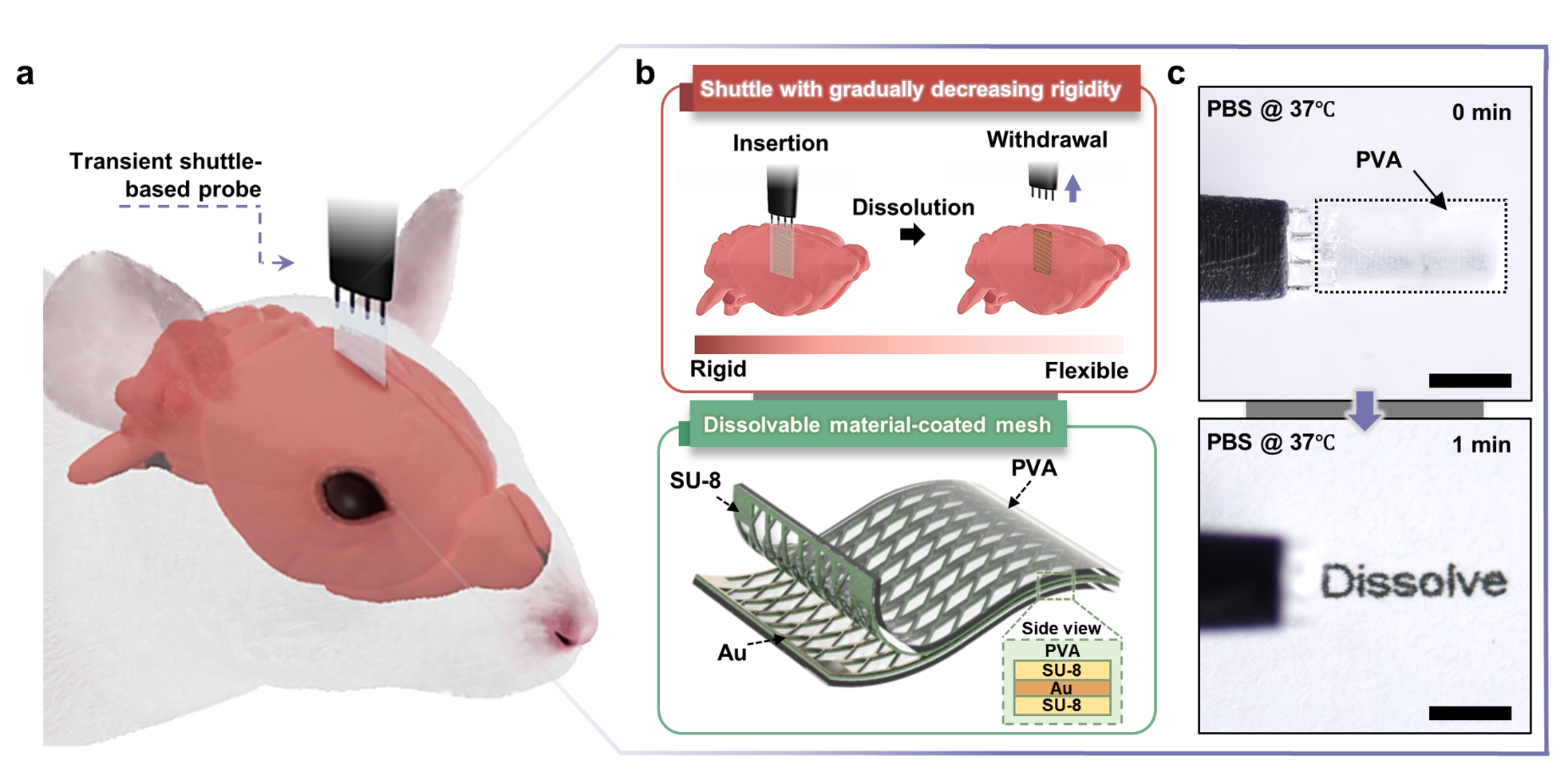
Our solution involves a PVA-based transient shuttle system. The PVA shuttle temporarily provides the necessary rigidity for the mesh probe to penetrate the brain. Once the probe is in place, the PVA shuttle dissolves, leaving behind an ultrathin (10 µm) flexible mesh electrode. This transition from high bending stiffness (3.59 nN∙m²) to low bending stiffness (3.33 pN∙m²) minimizes tissue damage and mechanical stress.
The dissolvable PVA forms a sharp edge and a lubricant layer during insertion, reducing penetration damage and shear stress between the probe and brain tissue. As the shuttle fully dissolves, there is no need to withdraw it, preventing any additional damage or displacement of the probe.
Our experimental results demonstrated significant improvements in both insertion and post-insertion phases. The PVA shuttle facilitated the smooth insertion of the mesh probe, maintaining structural integrity until complete dissolution. Post-dissolution, the probe's stiffness decreased dramatically, resulting in less mechanical stress on brain tissue. Histological analysis revealed minimal gliosis around the probe, indicating a reduced immune response and better integration with brain tissue. Neurons were observed migrating towards the mesh electrodes, suggesting improved long-term stability and functionality.
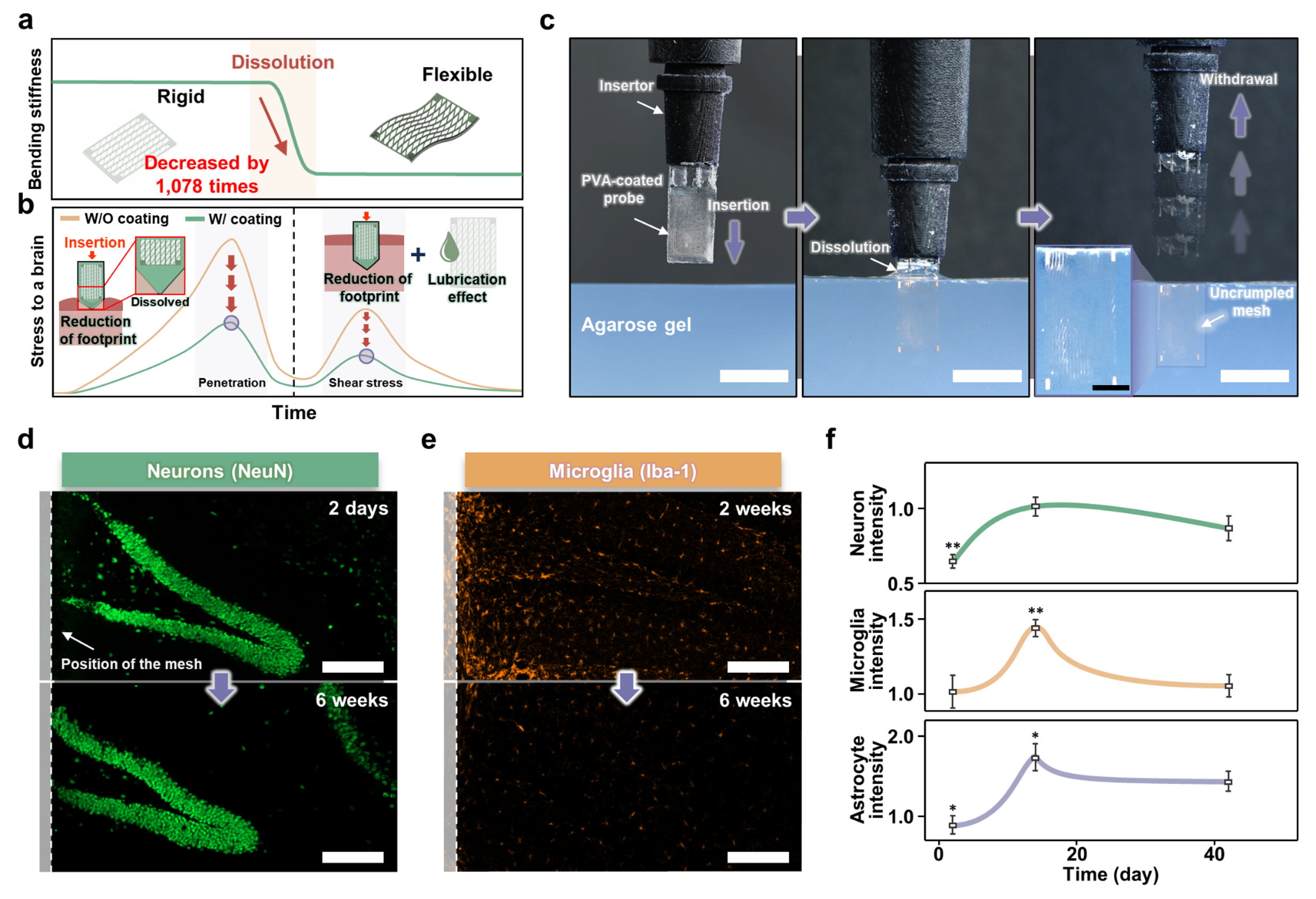
a, b, Schematic illustration and graph of the (a) stiffness change (before insertion: , after insertion: ) and (b) stress minimization to the brain through the decrease of the contact area and lubrication effect for inserting the transient shuttle-based probe. c, Sequential image of the process of implanting the probe into the agarose gel. d, e, Confocal fluorescence microscopy images of (d) neuronal and (e) microglial changes in brain tissue during implantation of the probe. f, Experimental intensity data for neurons and microglia within 0-25 mm proximity to the probe were recorded at intervals of 2 days, 2 weeks, and 6 weeks post-implantation (repeated on N=3 independent samples; all error bars represent mean±s.e.m.; independent two-sample t-test), and trend graphs were constructed based on the outcomes of the statistical significance tests. ; *p < 0.05, **p < 0.01 (Student's t test).
Mechanical stability tests further supported these findings, confirming that the probe remains safely in place within the brain despite its physiological movements. Tests conducted in agarose gel, simulating brain tissue, and in live brain tissue confirmed the probe's ability to adapt and maintain its position without causing additional damage.
In vivo experiments in mice demonstrated the probe's effectiveness under real biological conditions, with micro-CT imaging confirming accurate positioning and minimal displacement post-implantation. We were able to confirm through immunofluorescence staining that the insertion and withdrawal process prevents secondary damage, thereby reducing injury to the brain tissue. This results in a lower immune response.
The transient shuttle system represents a significant leap forward in neural interface technology. By providing temporary rigidity during insertion and subsequent flexibility, it addresses the primary challenge of mechanical mismatch between neural probes and brain tissue. This innovation opens up new possibilities for chronic neural monitoring and therapeutic applications, potentially revolutionizing the treatment of neurological disorders.
Follow the Topic
-
npj Flexible Electronics

This journal publishes high-quality papers related to flexible electronic systems, including plastic electronics and emerging materials, new device design and fabrication technologies, and applications.
Related Collections
With Collections, you can get published faster and increase your visibility.
Neuromorphic Circuits and Bio-inspired Systems
Publishing Model: Open Access
Deadline: Mar 31, 2026
Living and Biomaterials based Sustainable Flexible Electronics
Publishing Model: Open Access
Deadline: Aug 24, 2026






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in