Treating multiple myeloma with precision: treatment benefit of ixazomib-based therapy in patients with high-risk cytogenetic abnormalities
Published in Cancer

Multiple myeloma (MM) therapy has been revolutionized over the last two decades with the introduction of novel agents, such as immunomodulatory drugs, anti-CD38 antibodies, and proteasome inhibitors.1-3 Proteasome inhibitors have become a cornerstone of MM therapy,4 and their use in a continuous fashion, or to achieve higher cumulative doses, leads to improved long-term outcomes compared with fixed‑duration therapy.5-7 However, patient outcomes are impacted by various prognostic factors, such as cytogenetic abnormalities (CAs).8 Indeed, the survival of patients carrying certain CAs has generally remained poor compared with patients with standard-risk cytogenetics.3,9-11 Specific CAs associated with poorer prognosis include deletion of chromosome 17p [del(17p)], translocation between chromosomes 4 and 14 [t(4;14)], translocation between chromosomes 14 and 16 [t(14;16)], and amplification of 1q21 (amp1q21).3 Apart from amp1q21, these are all included in the 2016 International Myeloma Working Group (IMWG) definition of high-risk cytogenetics.12
It is well accepted among clinicians that proteasome inhibitors offer treatment benefits in patients carrying CAs.12 The results of several phase 3 studies have demonstrated the treatment benefit of ixazomib, an oral proteasome inhibitor, versus placebo-based therapy in patients carrying certain CAs.13-17 In TOURMALINE-MM1, relapsed, refractory, or relapsed and refractory MM (RRMM) patients with high-risk [del(17p) and/or t(4;14) and/or t(4;16)] CAs treated with ixazomib combined with lenalidomide-dexamethasone (IRd) had similar progression-free survival (PFS) to those with standard-risk cytogenetics versus patients who received placebo-Rd, suggesting that addition of ixazomib to Rd may have abrogated the increased risk conferred by CAs.16 Moreover, patients with high-risk and expanded high-risk (high risk and/or amp1q21) cytogenetics treated with IRd demonstrated treatment benefit versus placebo-Rd in the final overall survival analysis of TOURMALINE-MM1.17 In TOURMALINE-MM2, treatment with IRd led to improved PFS versus placebo-Rd in patients with newly diagnosed MM who had expanded high-risk cytogenetics.15 As a maintenance therapy, ixazomib has demonstrated a PFS benefit versus placebo in transplant-eligible patients with high-risk cytogenetics, and in transplant-ineligible patients with expanded high-risk cytogenetics in TOURMALINE-MM3 and -MM4, respectively.13,14 Collectively, these results support the treatment benefit of ixazomib in patients with high-risk and expanded high-risk cytogenetics.
With the evolution of individualized treatment and precision medicine in MM, there is an increasing need to understand how the efficacy of treatment options varies according to the presence of specific CAs.12,18 However, clinical trials assessing response to MM therapy according to specific CAs are limited,19 even though the IMWG have previously identified the analysis of cytogenetic subgroups in trials comparing different treatments as a key goal.12 By analyzing pooled data from four TOURMALINE studies (TOURMALINE-MM1, -MM2, -MM3, and -MM4), we evaluated the association between PFS and individual cytogenetic risk to assess whether certain patients benefit more from ixazomib-based therapy.13-16
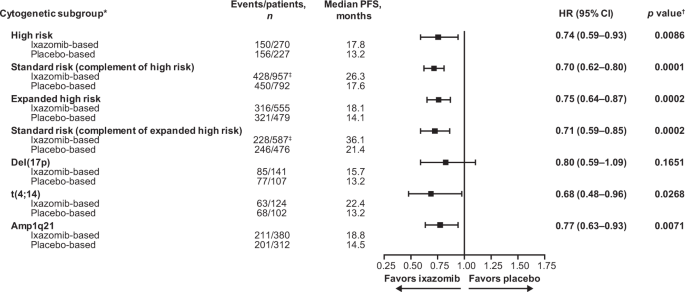
We showed a PFS benefit with ixazomib- versus placebo-based therapy in patients with MM regardless of the presence of specific CAs. After a median follow-up of 25.6 months, ixazomib prolonged median PFS by 4.6 and 4.0 months versus placebo-based therapy for patients with high-risk and expanded high-risk CAs, respectively. A similar magnitude of PFS benefit for ixazomib- versus placebo-based therapy was observed in patients with high-risk (hazard ratio [HR] 0.74) and expanded high-risk (HR 0.75) CAs compared to the respective complementary standard-risk subgroups (HRs 0.70 and 0.71).
We conducted further analyses of PFS according to the presence of specific CAs in the high-risk and expanded high-risk subgroups. Ixazomib prolonged median PFS by 9.2, 4.3, and 2.5 months versus placebo-based therapy in patients carrying t(4;14), amp1q21, and del(17p) CAs, respectively. These results highlight a greater PFS benefit of ixazomib- versus placebo-based therapy in patients carrying t(4;14) (HR 0.68) and amp1q21 (HR 0.77) CAs, suggesting that improved outcomes in the expanded high-risk subgroups were primarily driven by PFS differences in patients with these more frequent CAs. Although t(14;16) was also assessed, we did not draw conclusions from these data due to the small number of patients in the subgroup.
In TOURMALINE-MM1, patients received ixazomib in combination with Rd.16 As suggested by the IMWG,12 the triple-drug nature of this regimen may have influenced the ability of ixazomib to abrogate the negative impact of high-risk cytogenetics observed in this study.16,20 Our pooled analysis included two studies where patients received IRd (TOURMALINE-MM1 and -MM2), but also two studies where patients received single-agent ixazomib (TOURMALINE-MM3 and ‑MM4). Therefore, we conducted an analysis to assess the impact of single-agent ixazomib versus combination therapy. We identified a PFS benefit with ixazomib in combination with Rd compared with single-agent ixazomib in patients with high-risk CAs. The HR for PFS with ixazomib- vs placebo-based therapy was 0.64 for patients receiving ixazomib as combination therapy, and 0.83 for patients receiving single-agent ixazomib as maintenance therapy. Our results support the finding of TOURMALINE-MM1 that IRd is efficacious in patients with high-risk CAs and may improve the prognosis of these patients.16,20
As with other pooled analyses, ours was limited by differences in eligibility criteria, patient populations, and length of follow-up among the studies included. Additionally, we identified an imbalance in patient numbers between the standard-risk and high-risk subgroups, with more patients in the standard-risk subgroup. Further limitations included lack of uniformity in categorizing patients with additional copies of 1q as high-risk, and inconsistent cut-off values for defining the presence of high-risk CAs across the four studies. These limitations restricted the extent of cross-trial comparisons in this analysis.
Our findings demonstrate a PFS benefit for ixazomib- versus placebo-based therapy in patients with MM regardless of the presence of specific CAs. We observed a similar magnitude of PFS benefit in patients with high-risk and expanded-high-risk CAs compared to the complementary standard-risk subgroups. Of note, our results suggest that ixazomib- versus placebo-based therapy may provide a greater PFS benefit for patients carrying t(4;14) and amp1q21 CAs. We also report a PFS benefit for ixazomib in combination with Rd versus single-agent ixazomib in patients with high-risk MM. Overall, our findings indicate that ixazomib-based therapy may be a viable treatment option for patients with MM who have high-risk and expanded high-risk CAs, thus contributing to the evolution of precision medicine within MM.
REFERENCES
- Du J, Zhuang J: Major advances in the treatment of multiple myeloma in American Society of Hematology annual meeting 2020. Chronic Dis Transl Med 7:220-226, 2021
- Ito S: Proteasome Inhibitors for the Treatment of Multiple Myeloma. Cancers 12:1-19, 2020
- Rajkumar SV: Multiple myeloma: 2020 update on diagnosis, risk-stratification and management. Am J Hematol 95:548-567, 2020
- Kumar SK, Callander NS, Adekola K, et al: Multiple Myeloma, Version 3.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 18:1685-1717, 2020
- Jimenez-Zepeda VH, Duggan P, Neri P, et al: Bortezomib-containing regimens (BCR) for the treatment of non-transplant eligible multiple myeloma. Ann Hematol 96:431-439, 2017
- Mateos MV, Richardson PG, Dimopoulos MA, et al: Effect of cumulative bortezomib dose on survival in multiple myeloma patients receiving bortezomib-melphalan-prednisone in the phase III VISTA study. Am J Hematol 90:314-319, 2015
- Palumbo A, Gay F, Cavallo F, et al: Continuous Therapy Versus Fixed Duration of Therapy in Patients With Newly Diagnosed Multiple Myeloma. J Clin Oncol 33:3459-3466, 2015
- Wallington-Beddoe CT, Mynott RL: Prognostic and predictive biomarker developments in multiple myeloma. Journal of Hematology & Oncology 14:151, 2021
- D'Agostino M, Ruggeri M, Aquino S, et al: Impact of Gain and Amplification of 1q in Newly Diagnosed Multiple Myeloma Patients Receiving Carfilzomib-Based Treatment in the Forte Trial. Blood 136:38-40, 2020
- Goldschmidt H, Lokhorst HM, Mai EK, et al: Bortezomib before and after high-dose therapy in myeloma: long-term results from the phase III HOVON-65/GMMG-HD4 trial. Leukemia 32:383-390, 2018
- Jurczyszyn A, Charliński G, Suska A, et al: The importance of cytogenetic and molecular aberrations in multiple myeloma. Acta Haematologica Polonica 52:361-370, 2021
- Sonneveld P, Avet-Loiseau H, Lonial S, et al: Treatment of multiple myeloma with high-risk cytogenetics: a consensus of the International Myeloma Working Group. Blood 127:2955-2962, 2016
- Dimopoulos MA, Gay F, Schjesvold F, et al: Oral ixazomib maintenance following autologous stem cell transplantation (TOURMALINE-MM3): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet 393:253-264, 2019
- Dimopoulos MA, Spicka I, Quach H, et al: Ixazomib as Postinduction Maintenance for Patients With Newly Diagnosed Multiple Myeloma Not Undergoing Autologous Stem Cell Transplantation: The Phase III TOURMALINE-MM4 Trial. J Clin Oncol 38:4030-4041, 2020
- Facon T, Venner CP, Bahlis NJ, et al: Oral ixazomib, lenalidomide, and dexamethasone for transplant-ineligible patients with newly diagnosed multiple myeloma. Blood 137:3616-3628, 2021
- Moreau P, Masszi T, Grzasko N, et al: Oral Ixazomib, Lenalidomide, and Dexamethasone for Multiple Myeloma. N Engl J Med 374:1621-1634, 2016
- Richardson PG, Kumar SK, Masszi T, et al: Final Overall Survival Analysis of the TOURMALINE-MM1 Phase III Trial of Ixazomib, Lenalidomide, and Dexamethasone in Patients With Relapsed or Refractory Multiple Myeloma. J Clin Oncol 39:2430-2442, 2021
- Goldman-Mazur S, Vesole DH, Jurczyszyn A: Clinical implications of cytogenetic and molecular aberrations in multiple myeloma. Acta Haematologica Polonica 52:18-28, 2021
- Abdallah N, Rajkumar SV, Greipp P, et al: Cytogenetic abnormalities in multiple myeloma: association with disease characteristics and treatment response. Blood Cancer J 10:82, 2020
- Avet-Loiseau H, Bahlis NJ, Chng WJ, et al: Ixazomib significantly prolongs progression-free survival in high-risk relapsed/refractory myeloma patients. Blood 130:2610-2618, 2017
Follow the Topic
-
Blood Cancer Journal

This journal seeks to publish articles of the highest quality related to hematologic malignancies and related disorders.


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in