Treating space flight-induced bone loss: boldly going where no mouse has gone before
Published in Physics
Man has always of dreamed of traveling the heavens. But having evolved on Earth, with its gravitational forces, a strong limitation of extended space travel by humans is the negative effect of microgravity on the musculoskeletal system. Indeed, it has been observed that astronauts in low-Earth orbit lose up to 1% of their bone mass per month in space, that is about 24-fold more than people on Earth. Such extensive bone loss during space travel endangers the health of the astronauts during the trip but even long-term after they return.
Current strategies to mitigate such bone loss include exercise-induced mechanical loading during space travel. But so far this approach has proven to not be fully effective. Also, the time needed for such exercise limits the availability of the astronauts to perform their flight tasks, the equipment needed adds to valuable payload space and such an approach is contradicted for crew members that may be injured. To overcome these hurdles, a pharmacological approach that can be administered during flight may reduce the need for an exercise-related bone loss prevention program. In a new study published in npj Microgravity, Dr. Chia Soo, professor at UCLA Department of Surgery and UCLA Orthopaedic Surgery and Vice Chair of Research UCLA Plastic and Reconstructive Surgery and her co-senior authors report the effectiveness of one such pharmacological approach to prevent space travel-induced bone loss in rodents aboard the International Space Station (ISS).
Ideally, such a pharmacological approach should rely on a potent osteoinductive agent that only needs to be administered infrequently. One such molecule is the protein NELL-like molecule-1 (NELL-1). NELL-1’s osteoinductive property was initially identified by Dr. Kang Ting of the Forsyth Institute (Cambridge, MA) when he was affiliated with UCLA. Study co-senior author Dr. Ting showed that NELL-1 is highly expressed in active bone growth sites in a pathological condition called craniosynostosis, a development defect that is characterized by excess bone growth and premature fusion of the cranial sutures. Drs. Ting and Soo realized that the osteoinductive properties of NELL-1 could be leveraged to treat conditions involving excess bone loss such as osteoporosis or requiring more bone formation such as non-healing fractures.
However, there was a problem. NELL-1 therapy to treat rodent osteoporosis models required injection every 2 days. Fortunately, co-senior author, Dr. Benjamin Wu of the Forsyth Institute, formerly also affiliated with UCLA, had the perfect solution. Dr. Wu instructed his post doc and co-first author, Dr. Yulong Zhang, to not only PEGylate the protein to increase its half-life from 5.5 hours to 15.5 hours, but also to add a “smart” bone-targeting molecule, an inactivated bisphosphonate (BP) moiety, to the NELL-PEG compound. Various versions of NELL-PEG and BP-NELL-PEG were then tested in the Ting and Soo lab coordinated by co-first author and initial project manager, Dr. Jin Hee Kwak, on the extraordinarily complex task of getting the experiment ready for the Rodent Research – 5 (RR-5) mission. This included everything from making sure the identification microchips in the rodents did not migrate and interfere with DEXA (dual x-ray absorptiometry) scans to measuring bone density to in vitro and in vivo in Earth-bound studies to verify BP-NELL-PEG bioactivity, biodistribution, and efficacy using different doses of BP-NELL-PEG and different delivery routes (intravenous, subcutaneous, intraperitoneal). This was a once in a lifetime opportunity to work on the ISS—and failure was not an option! Ultimately, the combined team efforts led to a BP-NELL-PEG regimen that only needed to be delivered every two weeks intraperitoneally.
Using 80 adult female rodents, the team placed half of them on a SpaceX CRS-11 rocket bound for the ISS and kept the other half under the same housing conditions at the Kennedy Space Center in Florida as the Earth-bound “Ground Control.” (Figure 3 from the manuscript reproduced with permission). Of the 40 rodents on board the ISS, 20 were returned halfway through the 9-week study in the first ever Live Animal Return (LAR) in United States history. LAR is an exciting milestone that allows for accurate simulation of astronauts returning from space to Earth. National Aeronautics and Space Administration (NASA) Ames Research Center designed and engineered the special Rodent Research Hardware System to withstand the splash down force of the SpaceX Dragon into the Pacific Ocean. Amazingly, the LAR rodents were all alive and healthy—as testament to the great work at NASA Ames. The other 20 rodents were sacrificed at 9 weeks and returned via SpaceX CRS-12.
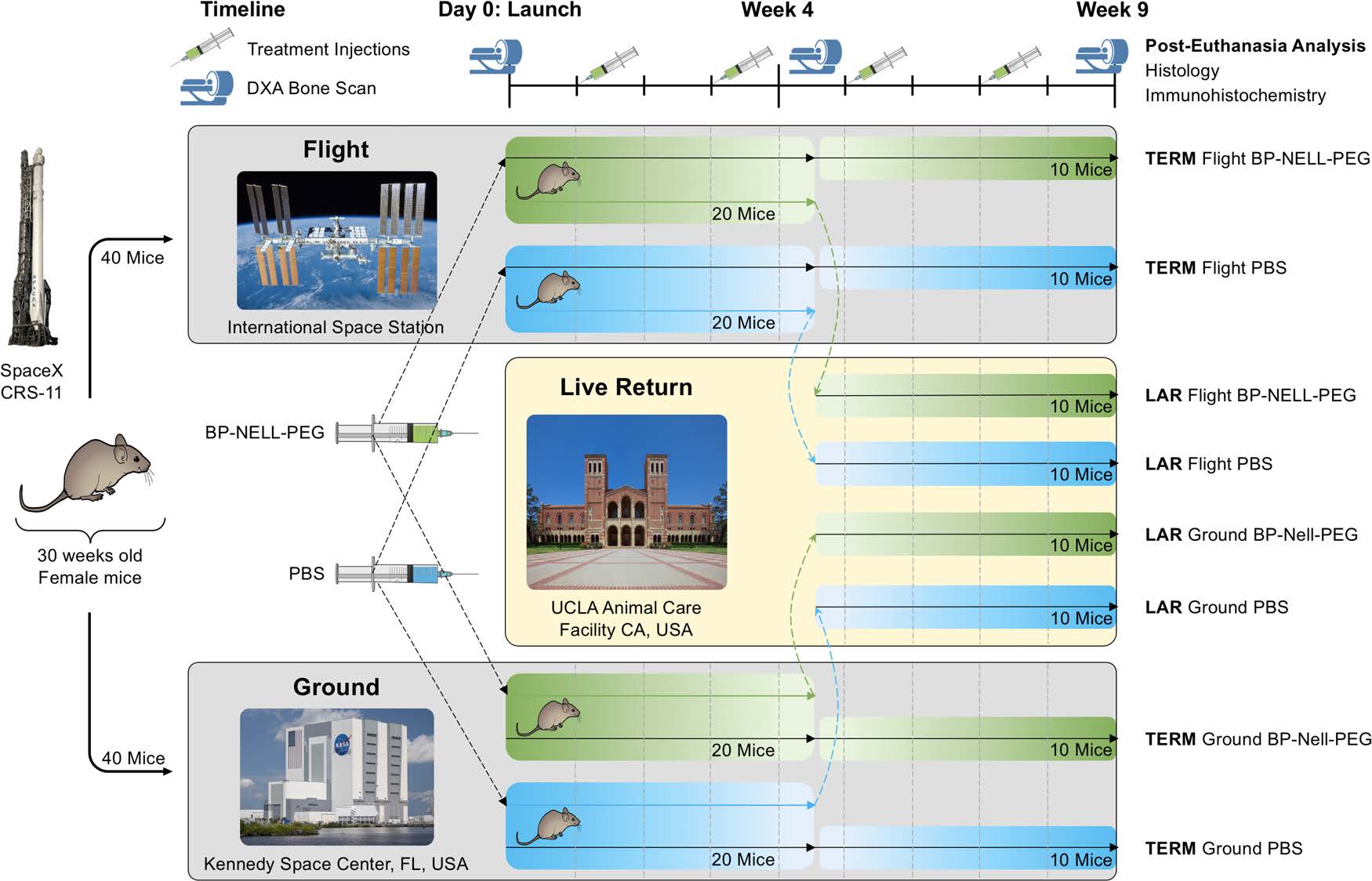
Back on Earth, co-first author and subsequent project manager, Dr. Pin Ha, UCLA Project Scientist in the Ting and Soo lab, coordinated analyses of the extensive amount of data. Helping Dr. Ha were many other co-authors, including Luan Tran and Dr. Timothy Pan Liu. Tran, a trail-blazing graduate student in Dr. Soo’s laboratory who is not only the first in his family to go to college, but is now striving for a PhD, performed much of the detailed imaging analyses. Dr. Liu is now a first year UCLA Orthopaedic Surgery resident. Dr. Liu, who will likely be a future star in orthopaedic surgery, worked closely with Dr. Ha and Tran to make the npj Microgravity manuscript come together.
Importantly, the team found that the BP-NELL-PEG-treated mice showed significantly improved bone mass compared to the PBS-treated controls, for both the Ground and Flight groups. This opens up the possibility that BP-NELL-PEG might one day be used to prevent bone loss during extended space flight—as well as treat osteoporosis or other pathological bone loss conditions on Earth. Future work includes finishing analyses of the LAR groups as well as exploring the use of BP-NELL-PEG in the clinic for bone loss here on Earth.
More information at: https://www.youtube.com/watch?v=wQa26sxshjk

Follow the Topic
-
npj Microgravity

This journal aims to provide a thorough understanding of the scientific impact and future of spaceflight research.
Related Collections
With Collections, you can get published faster and increase your visibility.
Space Biomanufacturing
Publishing Model: Open Access
Deadline: Mar 15, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in