TRIM69 suppressed the anoikis resistance and metastasis of gastric cancer through ubiquitin‒proteasome-mediated degradation of PRKCD
Published in Cancer
One of the primary challenges in the pursuit of an efficacious cancer treatment lies in combating the resilience of tumor cells to endure diverse stressful circumstances. This inherent capability facilitates the facile adaptation of cancer cells to disparate scenarios, thereby fostering the advancement and proliferation of tumors, ultimately serving as the principal catalyst for tumor resurgence. Consequently, the quest for effective molecular targets to surmount these constraints constitutes the paramount objective of numerous researchers worldwide.
Tumor metastasis is a multistep biological process in which cancer cells disperse from a primary site; cross the basement membrane (BM), the extracellular matrix (ECM) and vessel walls; and finally colonize distant organs through the blood and lymphatic circulation. Anoikis is a form of apoptosis resulting from loss of cell adhesion or adhesion-mediated signaling. Anoikis resistance is essential for tumor metastasis, allows tumor cells to survive during circulation in the bloodstream and facilitates their metastasis to distant organs. Previous studies have shown that tumor cells can avoid anoikis by inducing epithelial-mesenchymal transition (EMT), inhibiting apoptotic signaling pathways, changing integrin expression, regulating microRNAs, activating autophagy or inducing oxidative stress.
Our work established a model of anoikis resistance in gastric cancer (GC) cells in vitro and found that TRIM69 expression was significantly downregulated in anoikis-resistant GC cells. We confirmed that TRIM69 overexpression inhibited; while TRIM69 knockdown promoted; the anoikis resistance, migration and invasion of GC cells in vitro. Moreover, TRIM69 overexpression impaired distant metastasis in vivo. In addition, TRIM69 was expressed at low levels in metastatic GC tissues, and TRIM69 expression was negatively associated with distant metastasis. Although the mechanism by which TRIM69 is downregulated in anoikis-resistant GC cells remains unknown and needs further investigation, our data demonstrated a crucial role for TRIM69 in regulating anoikis resistance and metastasis in GC.
Ubiquitination is a fundamental, multifunctional posttranslational modification that plays a critical role in cellular processes, often leading to proteasomal degradation of binding proteins. E3 ubiquitin ligase regulates almost all cell processes, including cell cycle, transcription, DNA repair, signal transduction, endocytosis, cell transport and development, and can modulate the growth or apoptosis of tumor cells. Because of their large number, they represent a potential set of "Drug" targets. Therefore, E3 ligase small molecule therapeutics may have potential advantages in the treatment of cancer.
In the current study, the results of co-IP and LC‒MS/MS analysis showed that TRIM69 might interact directly with the PRKCD protein in GC cells. Importantly, PRKCD expression was regulated by TRIM69-mediated ubiquitination. Moreover, the results of the mutagenesis and co-IP assays showed that the ubiquitination of PRKCD induced by TRIM69 was both K48- and K63-linked, which are commonly observed types of ubiquitination. These results were consistent with a previous study reported by Han et al.[1]. More importantly, the rescue experiment showed that PRKCD overexpression abolished the effect of TRIM69 on GC cell anoikis resistance and metastasis in vitro and in vivo. Overall, we arrived at the novel conclusion that TRIM69 destabilizes PRKCD by enhancing its ubiquitination and degradation and suppresses the anoikis resistance and metastasis of GC cells through this effect on PRKCD.
Cytokines are key proteins in signaling in the tumor microenvironment (TME) that have pleiotropic effects and are associated with cancer progression, invasion, and metastasis. In this study, we found that TRIM69 inhibited BDNF expression in GC cells in a PRKCD-dependent manner. Additionally, treatment with hr BDNF abolished the TRIM69 overexpression-induced decrease in anoikis resistance and metastasis abilities of anoikis resistant GC cells. Altogether, these findings indicate that the roles of the TRIM69/PRKCD axis in preventing anoikis resistance and metastasis are dependent on BDNF. Importantly, we also identified a TRIM69-PRKCD+BDNF+ tumor cell subset, whose presence was positively correlated with distant metastasis in GC patients.
In summary, we investigated the novel role of TRIM69 in anoikis resistance and metastasis in GC and revealed a new mechanism for the regulation of PRKCD expression by TRIM69-mediated ubiquitination and degradation. Furthermore, TRIM69/PRKCD signaling was found to suppress anoikis resistance and metastasis by inhibiting BDNF expression. Hence, it is of great value to develop therapeutic approaches targeting TRIM69 or blocking PRKCD/BDNF as potential GC interventions.
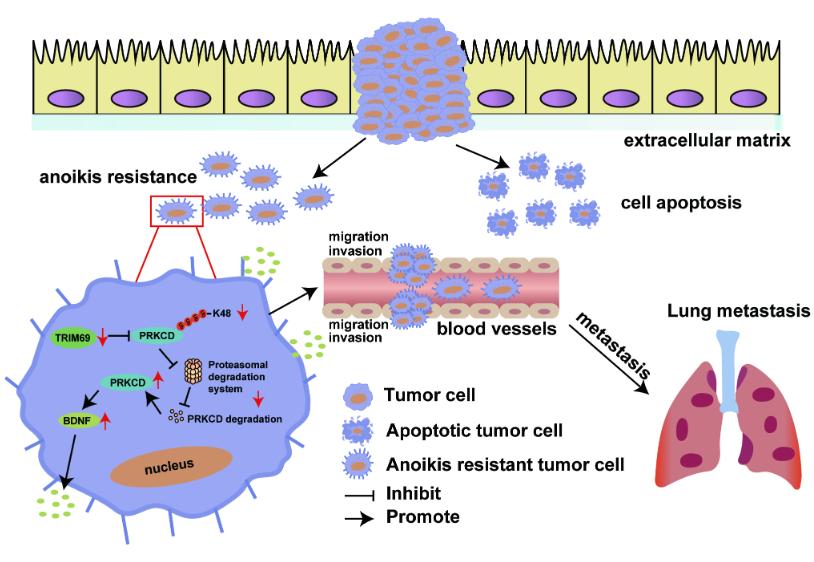
Figure 1 Schematic diagram showing the regulatory mechanisms of the TRIM69/PRKCD/BDNF axis in anoikis resistance and metastasis in GC. TRIM69 regulates the expression of BDNF mediated by PRKCD through K48 ubiquitin proteasome degradation pathway and participates in anoikis resistance and metastasis of gastric cancer.
References
[1] Han Y, Li R, Gao J, Miao S, Wang L. Characterisation of human RING finger protein TRIM69, a novel testis E3 ubiquitin ligase and its subcellular localisation. Biochemical and biophysical research communications 2012; 429: 6-11.
Follow the Topic
-
Oncogene

This journal aims to make substantial advances in our knowledge of processes that contribute to cancer by publishing outstanding research.



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in