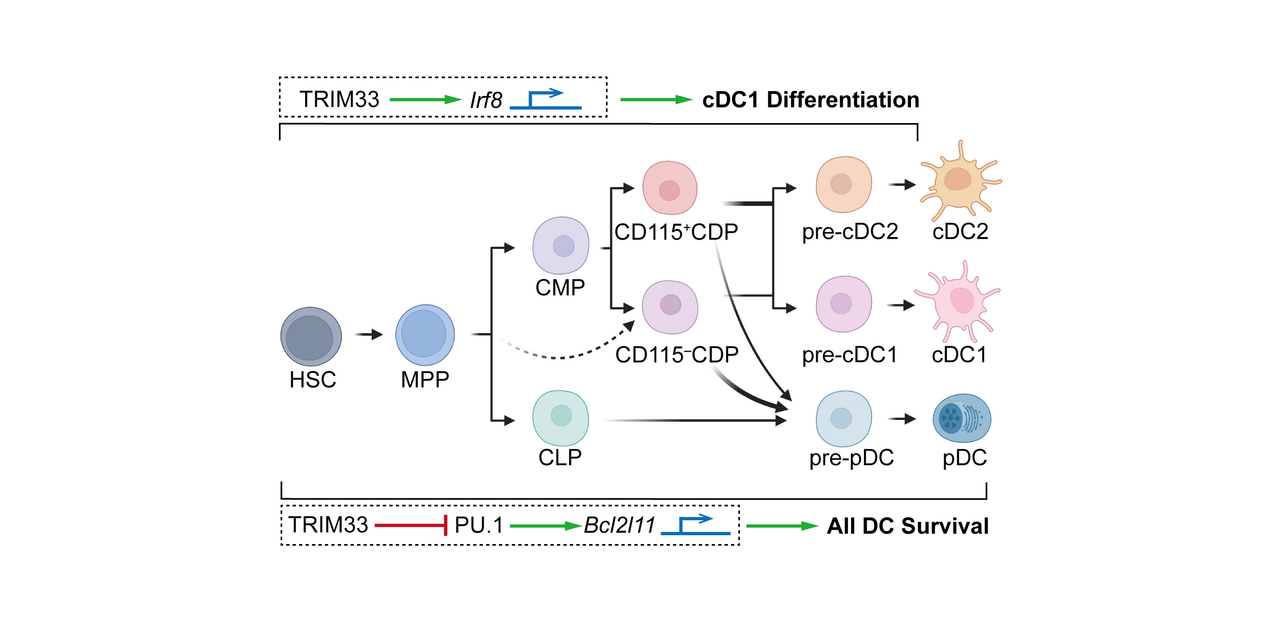
Our laboratory has long been focusing on the regulation of dendritic cell (DC) development. DC homeostasis is pivotal for appropriate innate and adaptive immunity1. In addition to the transcription factors and epigenetic modifiers we determined previously2-5, our current study identified TRIM33 as a critical regulator for DC differentiation and survival.
Although TRIM33 was known to influence the generation and function of erythroid, lymphoid, monocytic, and granulocytic lineages6-8, its elusive role in the DC lineage inspired our research. Moreover, our study was further motivated by the reported interaction8 between TRIM33 and PU.1, an essential DC fate regulator4, that implicated the potential participation of TRIM33 in DC homeostasis. To investigate the role of TRIM33 in DC homeostasis, we firstly phenotyped the Trim33fl/fl Cre-ERT2 mice with inducible systemic Trim33 deletion, and observed a striking absence of conventional type 1 DCs (cDC1s) and plasmacytoid DCs (pDCs), significant reduction of cDC2s, and profound disturbance of DC progenitor numbers and DC generation potentials. To further explain the molecular basis of such exciting findings, we then measured the expression of several key transcription factors and found a remarkably decreased expression of Irf8 in TRIM33-deficient DC progenitors. We further revealed a direct transcriptional regulation of Irf8 by TRIM33 by integrated mechanistic studies, which could account for the TRIM33-dependent differentiation of DCs, especially cDC1s. In addition, we identified hundreds of TRIM33 and PU.1 co-occupancy sites in DC progenitors, amongst them we revealed an enhancer site through which TRIM33 suppressed the transcription of the pro-apoptotic Bcl2l11(Bim) to prevent apoptosis of both DCs and their progenitors. Concurrently, we determined that CD11c+-conditioned Trim33 deficiency in Trim33fl/fl Itgax-Cre mice resulted in reduction in the numbers of most detected DC subsets due to impaired survival of terminally differentiated DCs. Furthermore, we demonstrated that simultaneous Irf8 overexpression and Bcl2l11 knockdown rescued cDC1 generation from TRIM33-deficient DC precursors, yet manipulation either alone could not do so. Thus, our finding identified new roles for TRIM33 in transcriptional regulation of both DC development and cell survival that collaboratively maintained DC homeostasis. Figure 1. below summarizes the crucial roles of TRIM33 in DC lineage homeostasis.
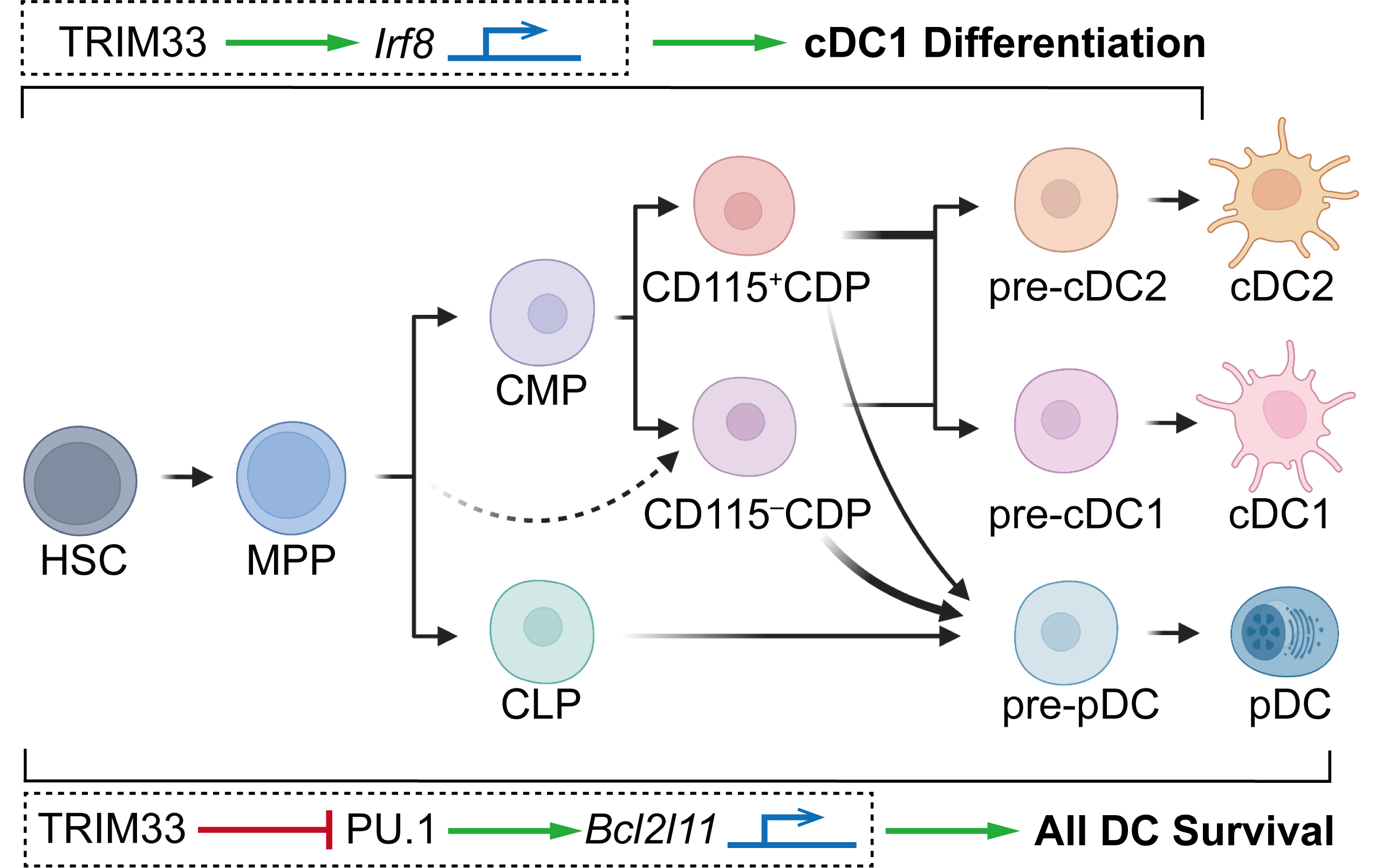
Fig. 1 TRIM33 is a crucial regulator for DC homeostasis.
We are also pleased that a paper just published in recent issue of Science Immunology9 also described a similar finding that further backed up our discovery. Since our work provided new knowledge on a potent transcriptional regulator of DC development and homeostasis, new strategies of DC-based immune modulations and therapies may benefit from this study.
References
1 Durai, V. & Murphy, Kenneth M. Functions of Murine Dendritic Cells. Immunity 45, 719-736, doi:https://doi.org/10.1016/j.immuni.2016.10.010 (2016).
2 Wu, L., Nichogiannopoulou, A., Shortman, K. & Georgopoulos, K. Cell-autonomous defects in dendritic cell populations of Ikaros mutant mice point to a developmental relationship with the lymphoid lineage. Immunity 7, 483-492 (1997).
3 Wu, L. et al. RelB is essential for the development of myeloid-related CD8 alpha(-) dendritic cells but not of lymphoid-related CD8 alpha(+) dendritic cells. Immunity 9, 839-847, doi:10.1016/s1074-7613(00)80649-4 (1998).
4 Carotta, S. et al. The Transcription Factor PU.1 Controls Dendritic Cell Development and Flt3 Cytokine Receptor Expression in a Dose-Dependent Manner. Immunity 32, 628-641, doi:10.1016/j.immuni.2010.05.005 (2010).
5 Zhang, Y. et al. Regulation of pDC Fate Determination by Histone Deacetylase 3. eLife 12, e80477, doi:10.7554/eLife.80477 (2023).
6 Rossmann, M. P. et al. Cell-specific transcriptional control of mitochondrial metabolism by TIF1gamma drives erythropoiesis. Science 372, 716-721, doi:10.1126/science.aaz2740 (2021).
7 Wang, E. et al. The transcriptional cofactor TRIM33 prevents apoptosis in B lymphoblastic leukemia by deactivating a single enhancer. Elife 4, e06377, doi:10.7554/eLife.06377 (2015).
8 Kusy, S. et al. Adult Hematopoiesis is Regulated by TIF1 gamma, a Repressor of TAL1 and PU.1 Transcriptional Activity. Cell Stem Cell 8, 412-425, doi:10.1016/j.stem.2011.02.005 (2011).
9 Tiniakou, I. et al. Genome-wide screening identifies Trim33 as an essential regulator of dendritic cell differentiation. Science immunology 9, eadi1023, doi:10.1126/sciimmunol.adi1023 (2024).
Follow the Topic
-
Cellular & Molecular Immunology

A monthly journal from the Chinese Society of Immunology and the University of Science and Technology of China, covering both basic immunology research and clinical applications.
Ask the Editor - Immunology, Pathogenesis, Inflammation and Innate Immunity
Got a question for the editor about the complement system in health and disease? Ask it here!
Continue reading announcement

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in