Tumor suppressor Par-4 activates autophagy-dependent ferroptosis
Published in Cancer and Cell & Molecular Biology
Cell death is a vital process that helps with the development and maintenance of organs, but it also plays a role in various human diseases. There are two main types of cell death: accidental (ACD) and regulated (RCD) [1]. Unlike accidental cell death, RCD is a controlled process with specific biochemical and genetic characteristics. RCD comes in different forms, such as apoptotic and non-apoptotic cell death. Among these, ferroptosis is a recently identified iron-dependent oxidative cell death mechanism that does not require the activation of apoptosis mediator caspases, highlighting the uniqueness of the execution of this death pathway [2]. Classical ferroptosis inducers, such as synthetic compounds like erastin and RSL3, were originally reported to inhibit the system Xc−-glutathione peroxidase 4 (GPX4) antioxidant axis, triggering lipid peroxidation in cancer cells [2]. Erastin and RSL3 can also cause iron accumulation during ferroptosis [2]. Various pathological conditions, including cancer, ischemic tissue injuries (e.g., ischemic heart disease, brain damage, kidney failure, and lung injury), and neurodegenerative diseases (e.g., Parkinson’s, Huntington’s, and Alzheimer’s diseases), exhibit increased ferroptotic activity [3]. Consequently, ferroptosis is considered a promising therapeutic target for these conditions.
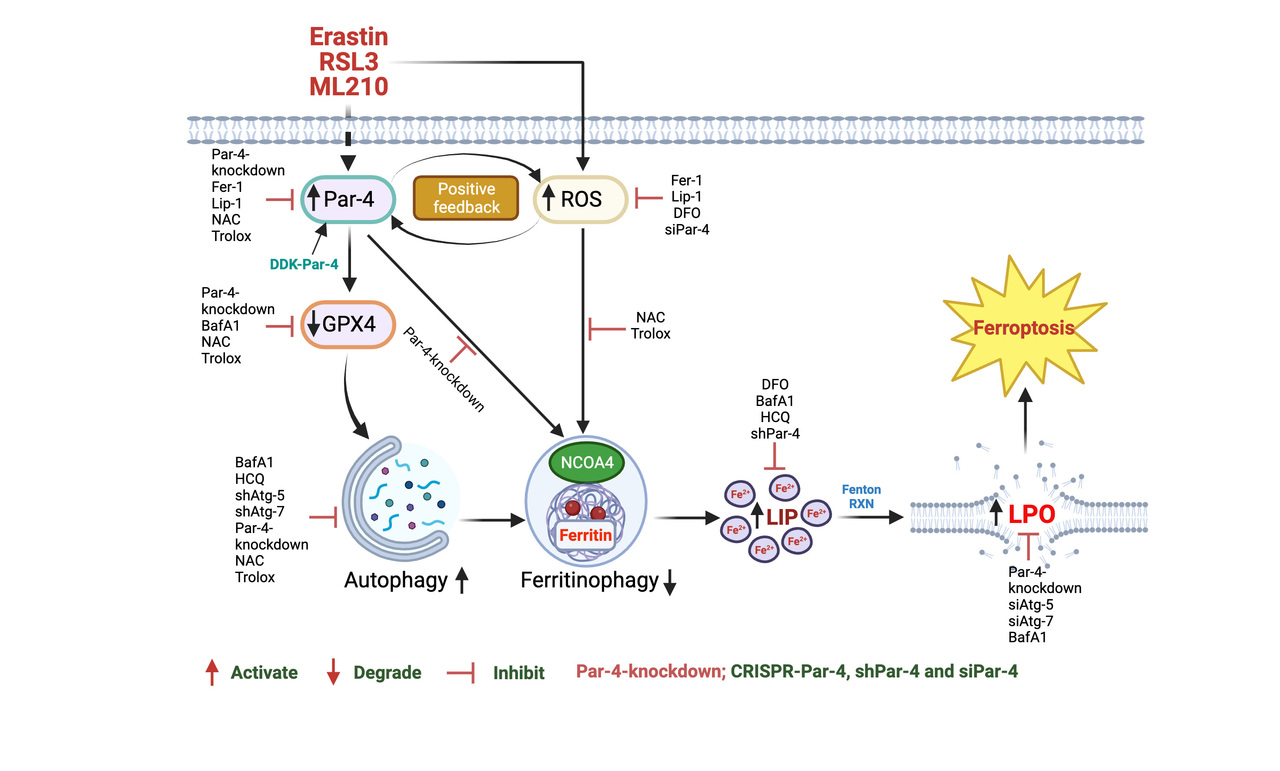
The interplay between autophagy and ferroptosis is well-established. Understanding the regulation of autophagy-dependent ferroptosis is of paramount importance. Recent studies have shown that ferroptosis activators increase autophagy flux, and cells lacking autophagy are resistant to ferroptotic damage or death [4]. Our recent findings indicate that acid sphingomyelinase, a crucial enzyme in sphingolipid metabolism, is involved in the autophagic degradation of GPX4, a key enzyme that inhibits ferroptosis [3]. These results highlight the significance of our research in developing therapeutic strategies that target ferroptosis to suppress tumors exhibiting high autophagy levels. Mechanistically, autophagy facilitates ferroptosis by degrading anti-ferroptotic proteins, such as ferritin. This autophagic process, termed ferritinophagy, elevates cellular ferrous iron levels, which subsequently induces reactive oxygen species (ROS) production and lipid peroxidation via the Fenton reaction [5]. Despite these advancements, the precise mechanisms and regulatory pathways governing autophagy-dependent ferroptosis remain inadequately understood.
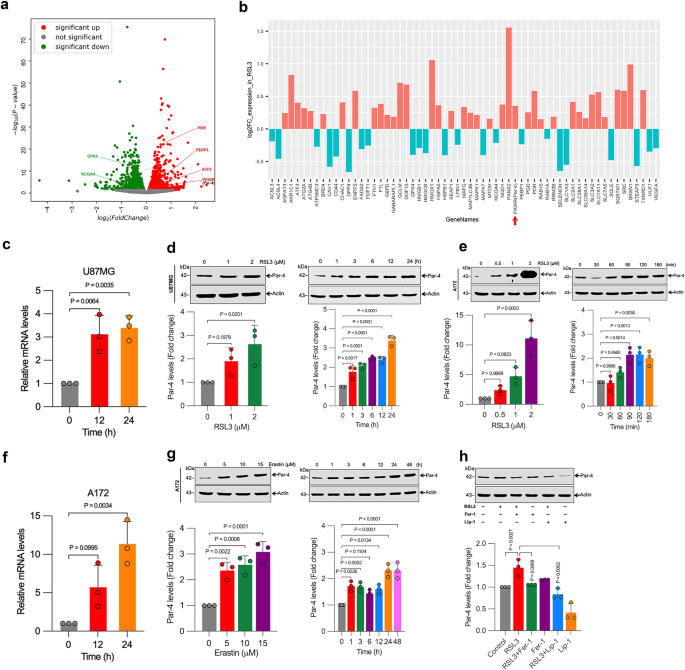
Prostate apoptosis response-4 (Par-4), encoded by the PAWR gene, is a key tumor-suppressor protein that selectively induces apoptosis in cancer cells [6]. Par-4 is ubiquitously expressed across various species but is often depleted, mutated, or inactivated in numerous cancers. For example, the loss or mutation of the PAWR gene on chromosome 12q21 is associated with Wilms’ tumor and male germ cell tumors. Par-4 knockout mice develop spontaneous tumors, whereas transgenic mice overexpressing Par-4 resist both spontaneous and oncogene-induced tumors [6]. Previous studies, including those from our laboratory, have shown that Par-4 can also induce autophagy [7,8], senescence [9], and anti-metastatic [10] processes. This highlights the diverse tumor-suppressive mechanisms of Par-4. Given our laboratory’s focus on both Par-4 and ferroptosis [3,11,12], we aim to explore the potential connections between these pathways for cancer therapy. An RNA-seq screen revealed significant up-regulation of Par-4 among 638 up-regulated and 594 down-regulated genes following treatment with the ferroptosis activator RSL3. This finding prompted us to investigate Par-4's role in enhancing the ferroptotic response in cancer cells, potentially leading to improved cancer treatments.
In our paper published in Communications Biology, we explored the role of the tumor suppressor Par-4 in autophagy-dependent ferroptosis. We demonstrated that genetic depletion of Par-4 via siRNA, shRNA, and CRISPR-Cas9 effectively inhibits ferroptosis, while Par-4 overexpression sensitizes cells to ferroptosis. Mechanistically, Par-4 promotes ferritinophagy through the nuclear receptor co-activator 4 (NCOA4), resulting in increased release of labile iron, enhanced lipid peroxidation, and concomitant ferroptosis. Inhibition of Par-4 disrupts the NCOA4-mediated ferritinophagy pathway. Additionally, Par-4 activation correlates with elevated reactive oxygen species (ROS) production, which is essential for ferritinophagy-mediated ferroptosis. Our results further show that Par-4 knockdown blocks ferroptosis-mediated tumor suppression in mouse xenograft models.
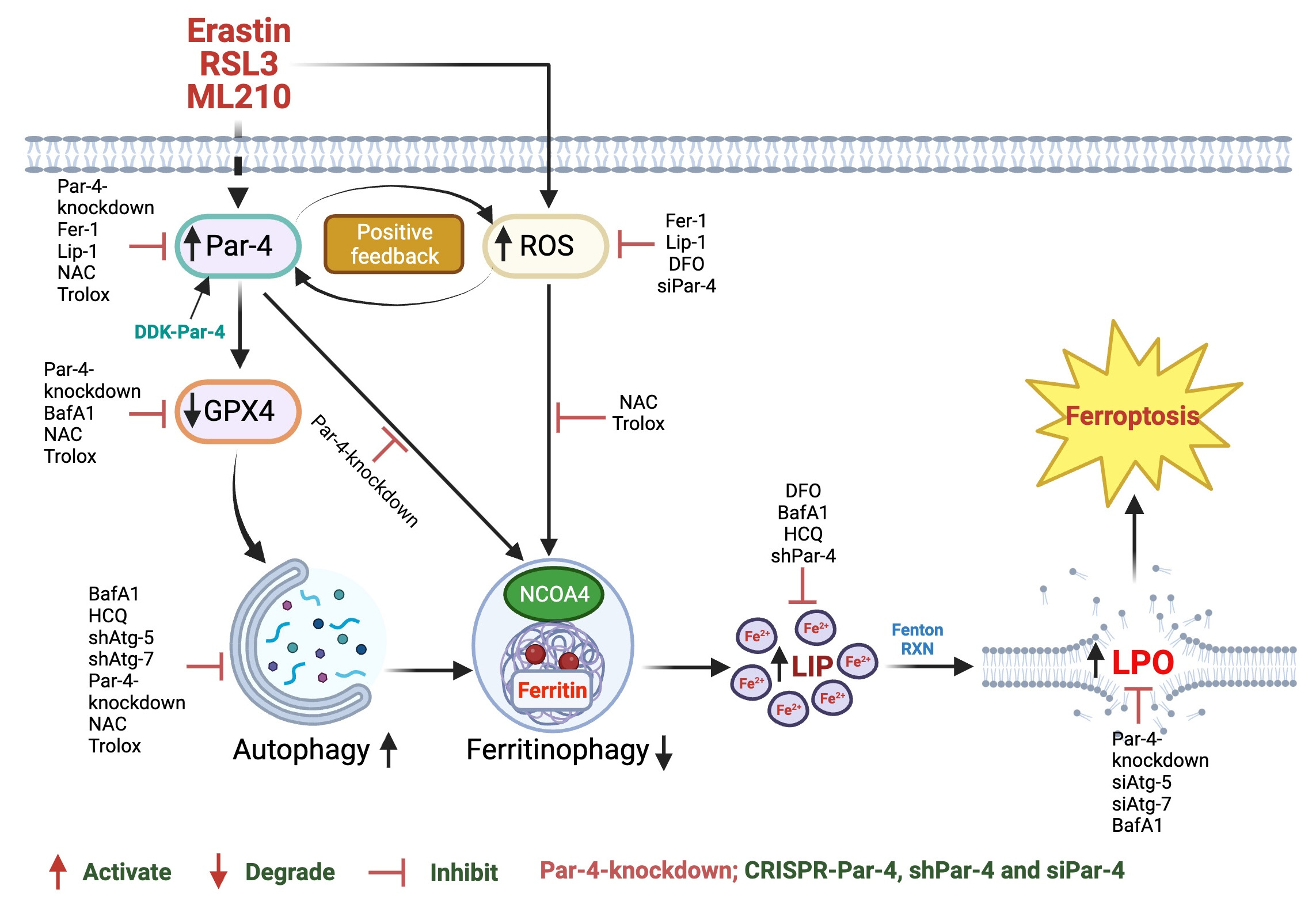
Figure 1. Schematic representation of Par-4-dependent ROS accumulation plays a critical role in RSL3, ML210-and erastin-induced autophagy-dependent ferroptosis in human glioblastoma cells
Our findings highlight the crucial role of Par-4 in ferroptosis and its potential as a novel therapeutic target in cancer therapy. Enhancing ferroptotic response in cancer cells via Par-4 presents a promising avenue for future cancer treatments.
Written by Karthikeyan Subburayan, Faisal Thayyullathil, and Sehamuddin Galadari.
References
- Tang D, Kang R, Berghe TV, Vandenabeele P, Kroemer G. The molecular machinery of regulated cell death. Cell Res. 29, 347-364 (2019).
- Dixon SJ, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 149, 1060-1072 (2012).
- Thayyullathil F, et al. Acid sphingomyelinase-dependent autophagic degradation of GPX4 is critical for the execution of ferroptosis. Cell Death Dis 12, 26 (2021).
- Liu J, Kuang F, Kroemer G, Klionsky DJ, Kang R, Tang D. Autophagy-Dependent Ferroptosis: Machinery and Regulation. Cell Chem Biol 27, 420-435 (2020).
- Hou W, et al. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy 12, 1425-1428 (2016).
- Cheratta AR, Thayyullathil F, Pallichankandy S, Subburayan K, Alakkal A, Galadari S. Prostate apoptosis response-4 and tumor suppression: it's not just about apoptosis anymore. Cell Death Dis 12, 47 (2021).
- Thayyullathil F, Rahman A, Pallichankandy S, Patel M, Galadari S. ROS-dependent prostate apoptosis response-4 (Par-4) up-regulation and ceramide generation are the prime signaling events associated with curcumin-induced autophagic cell death in human malignant glioma. FEBS Open Bio 4, 763-776 (2014).
- Thayyullathil F, et al. Par-4 regulates autophagic cell death in human cancer cells via upregulating p53 and BNIP3. Biochim Biophys Acta Mol Cell Res 1867, 118692 (2020).
- Subburayan K, Thayyullathil F, Pallichankandy S, Rahman A, Galadari S. Par-4-dependent p53 up-regulation plays a critical role in thymoquinone-induced cellular senescence in human malignant glioma cells. Cancer Lett 426, 80-97 (2018).
- Rasool RU, et al. A journey beyond apoptosis: new enigma of controlling metastasis by pro-apoptotic Par-4. Clin Exp Metastasis 33, 757-764 (2016).
- Subburayan K, Thayyullathil F, Pallichankandy S, Cheratta AR, Galadari S. Superoxide-mediated ferroptosis in human cancer cells induced by sodium selenite. Transl Oncol 13, 100843 (2020).
- Alakkal A, Thayyullathil F, Pallichankandy S, Subburayan K, Cheratta AR, Galadari S. Sanguinarine induces H2O2-dependent apoptosis and ferroptosis in human cervical cancer. 10, 1795 (2022).
Follow the Topic
-
Communications Biology

An open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of the biological sciences, representing significant advances and bringing new biological insight to a specialized area of research.
Your space to connect: The Cancer in understudied populations Hub
A new Communities’ space to connect, collaborate, and explore research on Cancers, Race and Ethnicity Studies and Mortality and Longevity!
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
Signalling Pathways of Innate Immunity
Publishing Model: Hybrid
Deadline: Feb 28, 2026
Forces in Cell Biology
Publishing Model: Open Access
Deadline: Apr 30, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in