Turning nitrate to ammonia via direct 8-electron electrochemistry
Published in Electrical & Electronic Engineering
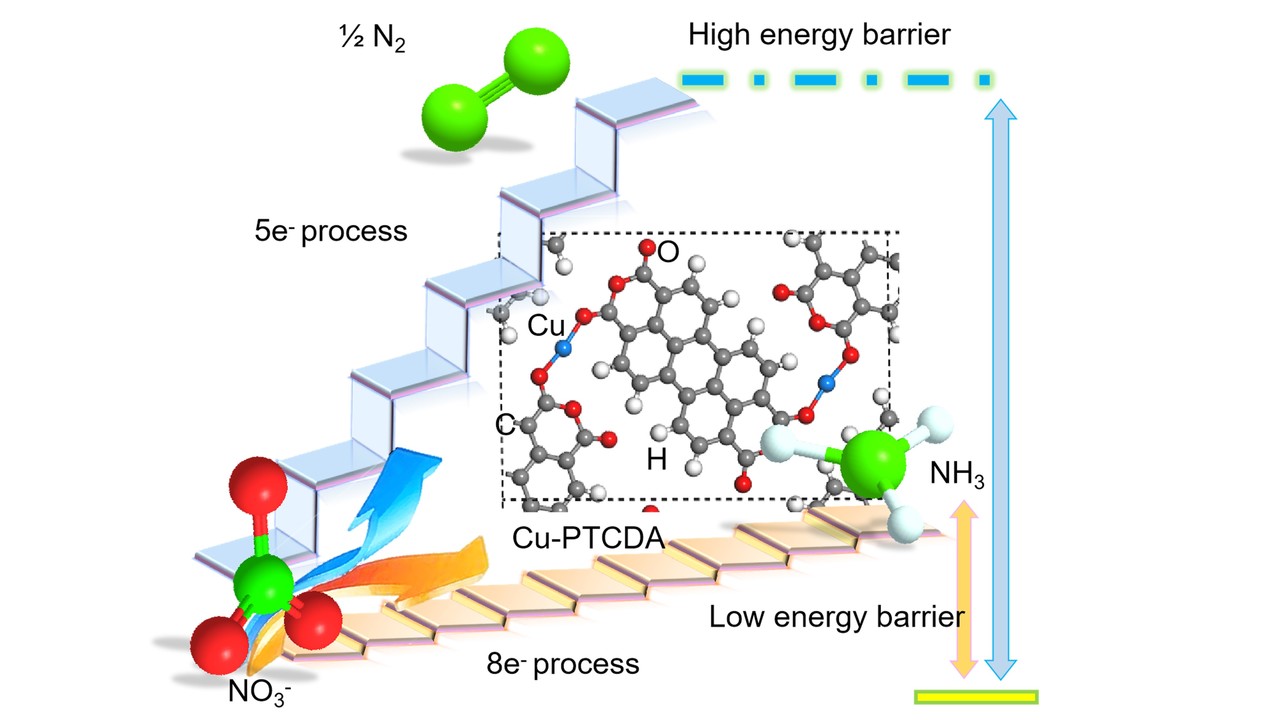
Playing a critical role in the fields of modern agriculture, industry and energy utilization, mass production of NH3 has been traditionally enabled by the well-known Haber–Bosch process under high temperature and pressure. Yet, the high-energy input and corresponding cost of the process have fueled much research into N2−H2O reactions under ambient conditions, preferably using renewable energy. Desirable merits in this low-cost process have unfortunately been voided by its low yield rate and poor selectivity, which is ascribed to the inert N≡N bond and ultralow solubility of N2 in H2O. Therefore, obtaining NH3 directly from non−N2 sources is considered as a revolutionary strategy to address these concerns. Here, an eight-electron direct reduction of NO3− to NH3, electrochemically catalyzed by a copper-incorporated 3,4,9,10−perylenetetracarboxylic dianhydride (Cu−PTCDA), is reported. The catalyst exhibits an NH3 production rate of 436±85 ug h-1 cm-2 and a maximum Faradaic efficiency of 85.9% at −0.4 V versus a reversible hydrogen electrode (RHE).
Since 2018, we have spent much time studying iron-organic solid catalyst to further improve the efficiency of electrochemical nitrogen reduction. The catalyst construction was inspired by the discovery of reductase with highly reactive metal centers and protein scaffolds regulating the transfer of protons and/or electrons and shunning hydrogen evolution reaction (HER). Unfortunately, no matter how we adjusted the reaction system, the performance didn’t improve much. The inert N≡N bond and ultralow solubility of N2 in electrolyte should be the cause of the limited performance.
Prior to this, we had noted that nitrogen reduction experiments could involve nitrogen contaminations (NOx), leading to false positive results. In this regard, we thought about whether NOx are actually easier to be reduced than N2. If so, NOx would be a suitable alternative nitrogen source to turn waste into treasure. In the search for alternatives, the NO3− stands out due to its rich abundance in nature as environmental pollutants. However, the conversion of NO3− into NH3 is not easily accessible. This process requires an eight-electron transfer reaction as well as a slightly lower reaction potential (1.20 V versus RHE) than that of the five−electron NO3−−to−N2 (1.25 V versus RHE). When we studied on iron–molecular organic solid catalyst for the NO3− reduction, it only showed a total Faradaic efficiency of 36.7%. Later, we screened over 20 metal elements as active centers and finally achieved a high selectivity of eight-electron NO3−−to−NH3 rather than the five-electron NO3−−to−N2 reaction (see the Figure) and HER suing copper–PTCDA catalyst. This work is an advance in the ammonia electro-synthesis under ambient conditions, and we expect to continue this non−N2 strategy to make further breakthrough in mass production of ammonia.
Follow the Topic
-
Nature Energy

Publishing monthly, this journal is dedicated to exploring all aspects of this on-going discussion, from the generation and storage of energy, to its distribution and management, the needs and demands of the different actors, and the impacts that energy technologies and policies have on societies.
Related Collections
With Collections, you can get published faster and increase your visibility.
Microgrids and Distributed Energy Systems
Publishing Model: Hybrid
Deadline: Mar 31, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in