Unanswered Questions: AML with IDH Mutations
Published in Cancer and General & Internal Medicine
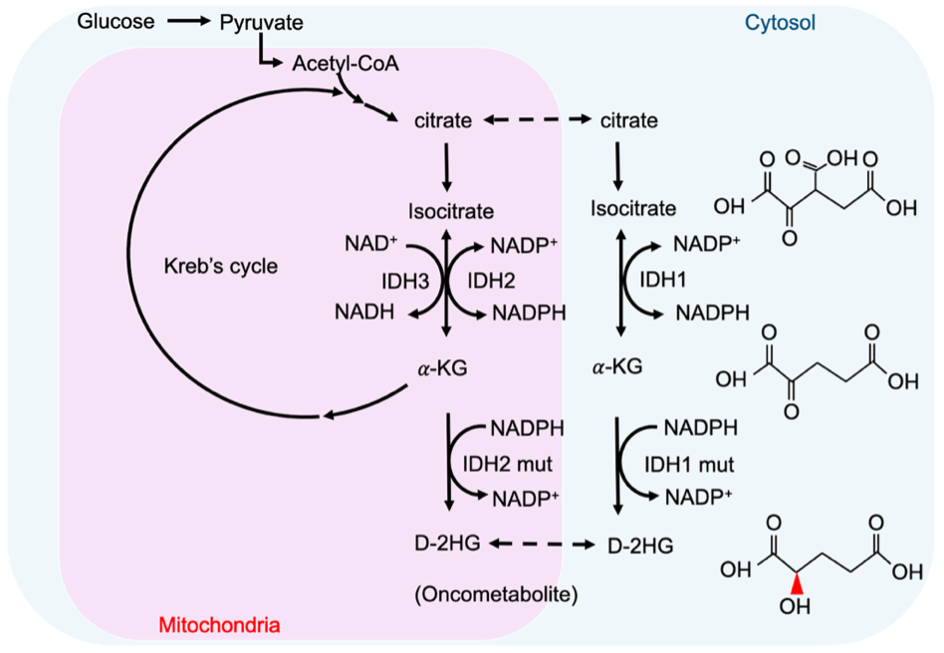
Acute myeloid leukemia (AML) is an aggressive cancer of the blood and bone marrow. Certain recurring genetic mutations can influence AML response to therapy and impact overall prognosis. One group of genetic mutations occurs in the IDH1 and IDH2 genes. These mutations are found in approximately 1 in 5 people newly diagnosed with AML. IDH mutations cause an accumulation of an abnormal cancer metabolite called 2-hydroxyglutarate, which interferes with normal gene regulation and can lead to the development of leukemia.
Two newer AML therapies have been shown to improve survival for patients with IDH-mutated AML. First are the IDH inhibitors ivosidenib and enasidenib, which specifically target the IDH1- and IDH2-mutated leukemia cells, respectively. Second is a drug called venetoclax (VEN), which promotes apoptosis of leukemia cells by inhibition of a molecule called BCL2. While these therapies have demonstrated efficacy in IDH-mutated AML, most of the current data pertain to relapsed/refractory AML or use in older adults who are ineligible for intensive chemotherapy (IC).
How can we improve treatment of IDH-mutated AML?
For younger patients with IDH-mutated AML, the current treatment standard remains IC; whether to add VEN and/or an IDH inhibitor is not well studied. There are some available data to guide our decision making, however. Stein et al (Blood 2021) showed that ivosidenib or enasidenib in combination with standard IC (7+3) resulted in robust remission rates in patients with IDH-mutated AML. Similarly, combinations of venetoclax-containing IC regimens (IC+VEN) have demonstrated outcomes in newly diagnosed AML patients, including a small subset of patients with IDH-mutated AML. Despite these data, the optimal induction regimen for IC-eligible patients with IDH-mutated AML has not been established.
To address this gap in knowledge, we analyzed the largest cohort to-date of patients with newly-diagnosed IDH-mutated AML who received frontline IC+VEN, with the goal of adding to the available data regarding outcomes of these patients in the contemporary treatment era.
How does IDH-mutated AML respond to IC+VEN?
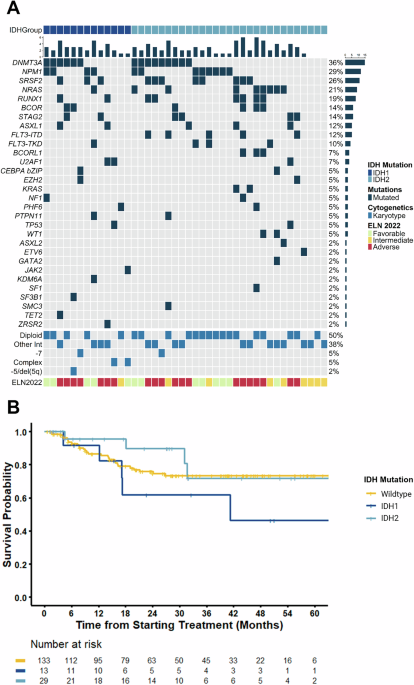
Our analysis showed that the addition of VEN to IC is effective and results in high rates of composite complete remission (CRc): 85% in IDH1- and 100% in IDH2-mutated AML. More impressively, IC+VEN resulted in high rates of measurable residual disease (MRD) negativity: 82% of IDH1-mutated and 89% of IDH2-mutated AML. With a median follow-up time of 30 months, patients treated with IC+VEN also had an impressive 3-year overall survival (OS) of 62% and 72% in IDH1- and IDH2-mutated AML, respectively.
However, there were some important differences noted depending on the type of IDH mutation present. Patients with IDH1 mutations had inferior response and survival rates compared to those with IDH2 mutations. This discrepancy may be driven, at least in part, by the higher frequency of adverse-risk clinical and molecular features in the IDH1-mutated cohort, including older age and unfavorable co-occurring genetic alterations.
To further examine this, we stratified IDH1-mutated patients based on the presence of additional genetic abnormalities. Notably, we found that there was a trend towards superior survival for IDH1-mutated AML with diploid karyotype (normal chromosomes), with a median OS at 3 years of 100% in the diploid group and 43% in the non-diploid group (p=0.081). There was no difference in median OS when IDH1-mutated patients were stratified by other genetic aberrations, although analysis was limited by the small number of patients in each group.
Patients with IDH1-mutated AML who went on to receive a stem cell transplant (SCT) had improved survival compared to those who did not, suggesting that this approach may mitigate some of the adverse outcomes associated with IDH1mutations. Specifically, IDH1 patients who received SCT (8 patients, 62%) had a median OS that was not reached versus 14.7 months in those who did not receive SCT (p=0.07). While not statistically significant, this trend warrants further investigation with larger cohorts.
What does this mean for patients?
Our findings demonstrate that IC+VEN is a highly active regimen in newly diagnosed AML with IDH mutations. However, IDH1-mutated AML continues to present therapeutic challenges, possibly due to the presence of additional high-risk features within their disease. This raises an important clinical question: should IDH inhibitors be added to IC regimens, with or without venetoclax, particularly for patients with IDH1-mutated AML? To answer this, further research in the form of randomized clinical trials is needed.
Above image sourced from: Chou FJ, Liu Y, Lang F, Yang C. D-2-Hydroxyglutarate in Glioma Biology. Cells. 2021 Sep 7;10(9):2345. doi: 10.3390/cells10092345.
Follow the Topic
-
Leukemia

This journal publishes high quality, peer reviewed research that covers all aspects of the research and treatment of leukemia and allied diseases. Topics of interest include oncogenes, growth factors, stem cells, leukemia genomics, cell cycle, signal transduction and molecular targets for therapy.





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in