Unconventional transmission mode of the delta virus
Published in Microbiology
Discovered 40 years ago in the liver of individuals chronically infected with HBV, HDV is a liver-specific human pathogen responsible for chronic liver diseases, with over 250 million infected individuals throughout the world. HDV is a satellite virus and belongs to a group of infectious agents that are related to plant viroids and that are completely distinct from HBV. Yet, HDV coinfects ca. 5-10% of HBV-infected persons, where it worsens HBV pathogenicity by accelerating the outcome and severity of hepatitis.
Satellite viruses are scarcely found in animal viruses in contrast to their profusion in plant viruses. Only two representative satellite viruses are known currently in human viruses and include HDV and adeno-associated virus (AAV), which uses helper functions of e.g. adenovirus or herpes simplex virus at the level of replication of its genome, unlike for HDV. Indeed, HDV is defective for transmission of its RNP and relies on HBV surface glycoproteins (GPs) to induce envelopment and secretion of its RNA genome as well as transmission to other cells via HBV cell entry factors.
The HDV genome is unique among animal viruses; yet, we were intrigued that HDV can efficiently replicate in different tissues and species, though in nature, it seems restricted to the human liver. Therefore, we raised the hypothesis that HDV may have arisen from and/or conceivably still infect hosts independently of HBV. It also happens that our lab has focused much of its research interests in understanding the intense flexibility of alternative RNA viruses to use surface GPs from other enveloped viruses (1), a mechanism called pseudotyping that is widely exploited to generate retroviruses and lentiviral vectors displaying the host-ranges of the GP donor virus (2) and that is very useful for multiple basic studies (3) as well as for therapeutic gene transfer (4). Thus, on this ground and aiming to explore scenarios concerning the origin of HDV, we decided to investigate the possibility that other, HBV-unrelated viruses could provide helper envelopment, budding, and entry functions.
Amazingly, we found that HDV RNPs may exploit assembly functions provided by viruses from several alternative genera and families, including vesiculovirus, flavivirus, and hepacivirus amongst others (5). This compatibility allows efficient egress in the extracellular milieu of co-infected cells of HDV particles that appear to be infectious (Figure 1). This leads to their subsequent entry into different cell types expressing the receptors targeted by the GPs of either virus genus and dissemination of HDV genome in vivoin infected humanized mice (5).
That unconventional cell transmission of HDV is experimentally possible in vivo raises the possibility that in nature, HDV could be associated with different virus types, including human viral pathogens, which could possibly favour previously unappreciated HDV transmission scenarios and modulate their pathogenicity. This may explain recent findings that viruses closely related to HDV have been detected in non-human species in the absence of any hepadnavirus (6, 7). Also, primary Sjögren’s syndrome patients were reported to present HDV antigen and RNA in salivary glands in absence of HBsAg or HBV antibodies (8). Overall, our demonstration that unconventional cell transmission of HDV is experimentally possible in vivo warrants that studies be conducted in infected individuals.
Reference:
Perez-Vargas, J., F. Amirache, C. Mialon, B. Boson, N. Freitas, C. Sureau., F. Fusil and F.-L. Cosset (2019). "Enveloped viruses distinct from HBV induce dissemination of hepatitis D virus in vivo." Nature Communications. https://rdcu.be/bAQHV
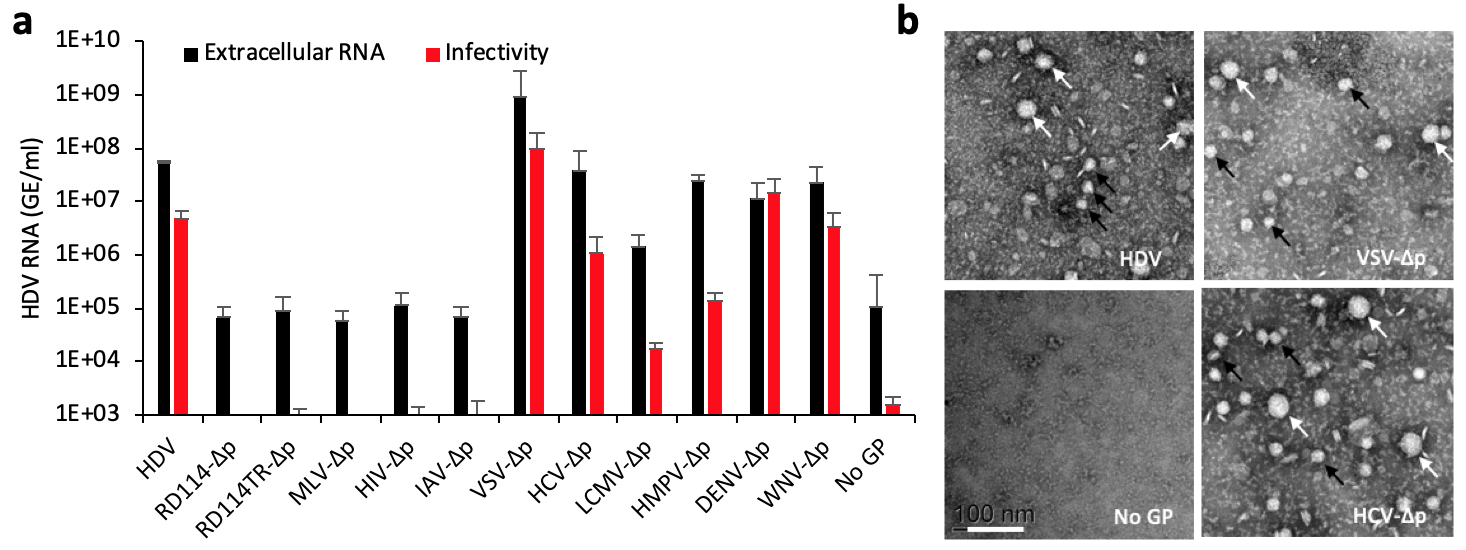
Figure 1. HDV particles generated with heterologous envelope glycoproteins are infectious. (a) Secretion and infectivity of delta particles (∆p) co-expressed with HBV GPs (designated “HDV”) or with surface GPs of the indicated viruses. As control, HDV was expressed without GPs (referred to as “No GP”), which provides baselines of HDV RNA RTqPCR analyses in producer Huh-7 cell supernatants (black bars) or in inoculated susceptible cells (red bars). The results are expressed per mL of cell supernatants or of cell lysates containing 1e6 cells. (b) Electron microscopy of the indicated heparin bead-purified ∆p particles as examined by electron microscopy samples after elution and negative staining. Note the presence of large (white arrows) and small (black arrows) particles. Scale bar (lower left): 100 nm.
Literature cited:
1. Zavada J.1982. The pseudotypic paradox. J Gen Virol 63:15-24.
2. Sandrin V, Cosset F-L.2006. Intracellular vs. cell surface assembly of retroviral pseudotypes is determined by the cellular localization of the viral glycoprotein, its capacity to interact with Gag and the expression of the Nef protein. J Biol Chem 281:528-542.
3. Bartosch B, Dubuisson J, Cosset FL.2003. Infectious hepatitis C virus pseudo-particles containing functional E1-E2 envelope protein complexes. J Exp Med 197:633-42.
4. Levy C, Verhoeyen E, Cosset FL.2015. Surface engineering of lentiviral vectors for gene transfer into gene therapy target cells. Curr Opin Pharmacol 24:79-85.
5. Perez-Vargas J, Amirache F, Mialon C, Boson B, Freitas N, Sureau. C, Fusil F, Cosset F-L.2019. Enveloped viruses distinct from HBV induce dissemination of hepatitis D virus in vivo. Nature Communications10:2098. https://rdcu.be/bAQHV
6. Hetzel U, Szirovicza L, Smura T, Prahauser B, Vapalahti O, Kipar A, Hepojoki J.2019. Identification of a Novel Deltavirus in Boa Constrictors. MBio 10:e00014-19.
7. Wille M, Netter HJ, Littlejohn M, Yuen L, Shi M, Eden JS, Klaassen M, Holmes EC, Hurt AC.2018. A Divergent Hepatitis D-Like Agent in Birds. Viruses 10:720.
8. Weller ML, Gardener MR, Bogus ZC, Smith MA, Astorri E, Michael DG, Michael DA, Zheng C, Burbelo PD, Lai Z, Wilson PA, Swaim W, Handelman B, Afione SA, Bombardieri M, Chiorini JA.2016. Hepatitis Delta Virus Detected in Salivary Glands of Sjogren's Syndrome Patients and Recapitulates a Sjogren's Syndrome-Like Phenotype in Vivo. Pathog Immun 1:12-40.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in