Understanding brain aging through multimodal diversity and inequality
Published in Neuroscience, Public Health, and Behavioural Sciences & Psychology
Every brain has its own rhythm, a biological clock that tracks not only the passage of time but also the influence of our environment and life circumstances. In our recent study, published in Nature Medicine, we explored how multimodal diversity can offer a unique perspective on understanding brain aging and neurocognitive disorders, especially in diverse and underrepresented populations.
Clinical conditions and accelerated brain aging
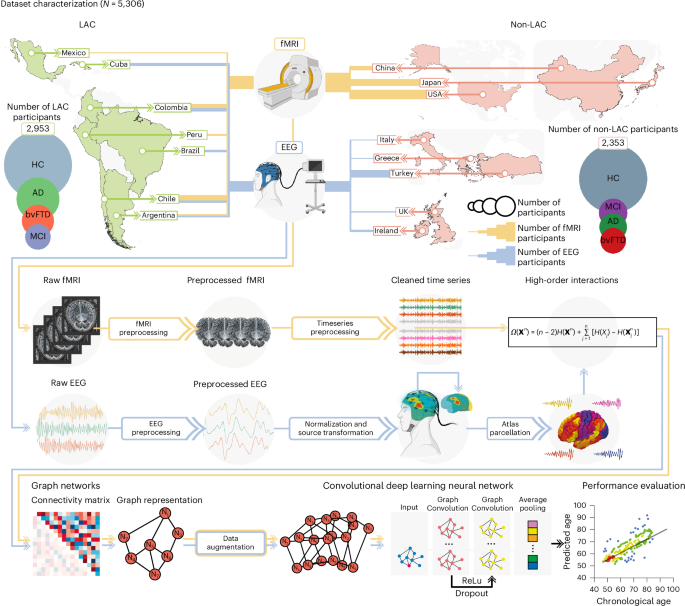
Using multimodal data from over 5,000 participants in 15 countries, including functional magnetic resonance imaging (fMRI) and electroencephalography (EEG), we developed brain-age gap models showing that brain aging speeds up progressively across groups of healthy individuals to those with neurocognitive disorders. Specifically, we observed that the brain-age gaps increase from healthy controls to those with mild cognitive impairment (MCI) to Alzheimer’s disease (AD) and behavioral variant frontotemporal dementia (Figure 1a and 1b). Participants with MCI had brain ages that fell between those of healthy aging and neurodegenerative conditions, suggesting that brain-age clocks could help identify individuals at higher risk of progressing to dementia.
Interestingly, we found that brain aging is influenced by individual clinical conditions and country aggregate-level socioeconomic factors and physical exposures. Individuals in countries with more socioeconomic inequalities, pollution, and limited healthcare (such as those in Latin America and the Caribbean, LAC), tend to have older brains. These findings highlight the importance of considering multimodal diversity and social determinants of health when assessing brain aging and dementia risk, particularly in regions with significant structural disparities.
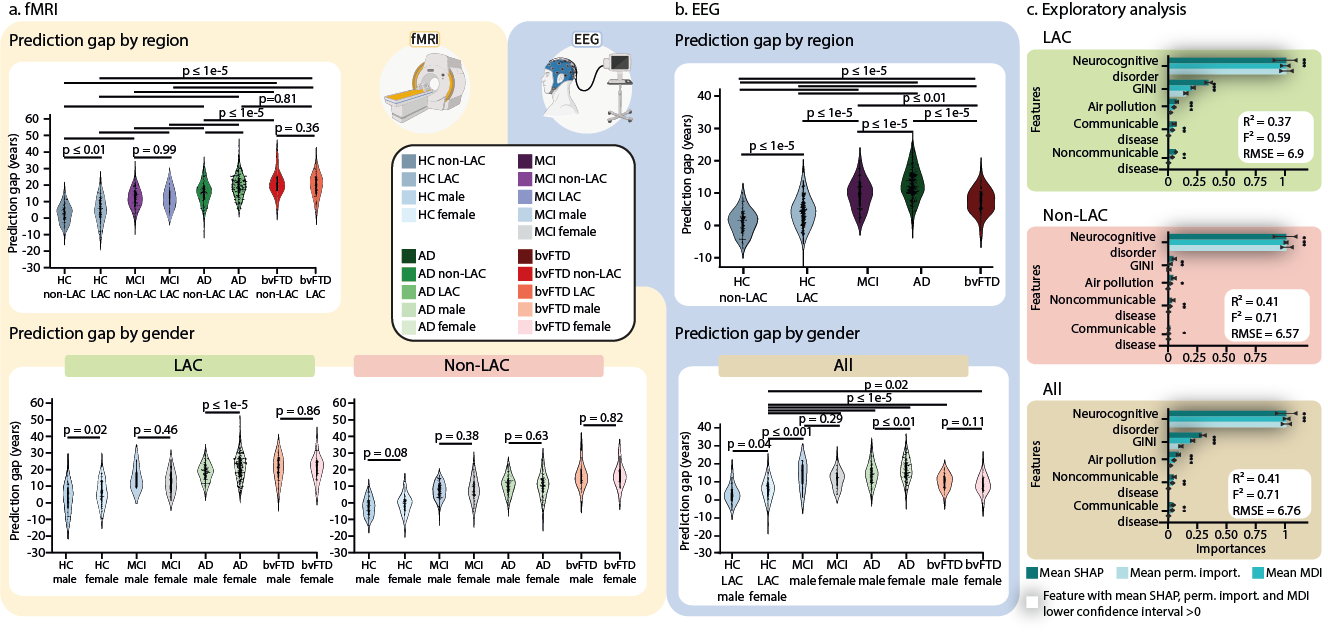
Fig. 4. Groups, sex, and macrosocial influences in brain-age gaps. Violin plots for the distribution of prediction gaps for different groups and sex effects using (a) fMRI and (b) EEG datasets. The statistical comparisons were calculated using two-sided subsample permutation testing without multiple comparisons and with 5000 algorithm iterations. HC non-LAC = Healthy controls from non-LAC, HC LAC = Healthy controls from LAC, MCI = mild cognitive impairment, AD = Alzheimer’s disease, bvFTD = behavioral variant frontotemporal dementia, M = males, F = females, * p < 0.05, ** p < 0.01, *** p < 0.001. (c) Associations between macrosocial and disease disparity factors with brain-age gaps were assessed with a multi-method approach comprising SHapley Additive exPlanations (SHAP) values, feature importance (mean decrease in impurity, MDI), and permutation importance. Plots show the mean importance values for each method, along with their 99% confidence interval, as well as the average R-squared and Cohen's f². * = Features whose lower confidence interval boundary does not cross zero. Shaded bars indicate significance across the three methods. We conducted a two-sided F-test to evaluate the overall significance of the regression models. The three models were significant: healthy controls LAC: R² = 0.37 (99% CI ±0.17), F² = 0.59 (99% CI ±0.21), RMSE = 6.9 (99% CI ±0.92), F = 138.78 ( p < 1e-15), healthy controls non-LAC: R² = 0.41 (99% CI ±0.17), F² = 0.71 (99% CI ±0.21), RMSE = 6.57 (99% CI ±1.31), F = 135.91 (p < 1e-15), and total dataset (R² = 0.41 (99% CI ±0.12), F² = 0.71 (99% CI ±0.14), RMSE = 6.76 (99% CI ±0.89), F = 253.39, (p < 1e-15). The relevance of the features and their respective confidence intervals are available in Supplementary Table 2. This figure was partially created with BioRender.com under individual license.
Gender inequality’s impact on brain health
One of the key findings from our study is the difference in brain aging between male and female individuals, especially in LAC. Female participants, both healthy and those with AD, showed accelerated brain aging compared to male individuals (Figures 1a and 1b). Gender inequality worsens brain aging for female participants. Despite progress, women in LAC still face challenges like lower education, less income, limited healthcare access, and greater caregiving responsibilities—all of which negatively impact brain health and raise the risk of AD. Sex-specific factors also play a role, as two-thirds of those with AD are female individuals. While they may show more cognitive resilience in the early stages, they often experience faster decline as the disease progresses. Biological differences, such as the effects of menopause—reducing brain volume and increasing harmful proteins linked to AD—also contribute. Sex hormones and chromosomes influence different AD mechanisms during aging, including inflammation, metabolism, and autophagy (the process by which cells clean out and recycle damaged or unnecessary parts), resulting in distinct patterns of disease progression between male and female. These biological and gender disparities underscore the need for tailored approaches to brain health and dementia, particularly in underserved regions.
The need for multimodal diversity in brain health research
Our study underscores the critical need for incorporating multimodal diversity, including clinical factors and gender inequality, into brain aging and brain health research. Many previous studies have focused on high-income countries, often overlooking how sex and gender differences manifest in other regions. Our findings emphasize the value of embracing diverse approaches to better understand the complex interactions between biology and the environment that influence brain health, especially in areas with significant inequality. By adopting diverse and inclusive research approaches, we can move toward personalized medicine models that truly reflect all populations.
For more information: Moguilner, S., Baez, S., et al. Brain clocks capture diversity and disparities in aging and dementia across geographically diverse populations. Nat Med (2024). https://www.nature.com/articles/s41591-024-03209-x
The poster image was created with GPT4 under the supervision of the authors.
Follow the Topic
-
Nature Medicine

This journal encompasses original research ranging from new concepts in human biology and disease pathogenesis to new therapeutic modalities and drug development, to all phases of clinical work, as well as innovative technologies aimed at improving human health.
Your space to connect: The Psychedelics Hub
A new Communities’ space to connect, collaborate, and explore research on Psychotherapy, Clinical Psychology, and Neuroscience!
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
Stem cell-derived therapies
Publishing Model: Hybrid
Deadline: Mar 26, 2026
Digital Medicine for Infectious Diseases
Publishing Model: Hybrid
Deadline: Nov 09, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in