Understanding the Health Impacts of Your Genetically Determined Testosterone Levels
Published in Healthcare & Nursing
Not many molecules have gained as much popularity as testosterone as a potential explanation for many different features in our physiology and everyday lives. When talking to a group of people, one can try combining testosterone and any trait showing male-female differences in the same sentence. Quite often “oh yes, it must be testosterone” you feel the room agree and see the nodding heads. Truly, the hormone is often used as a low hanging fruit to explain variation, e.g., in traits that show sex differences. But how much does normal variation in average adult testosterone levels actually contribute to differences in human traits?
Since its discovery in the early 20th century, testosterone has been connected to health, behavior and appearance in different species, including humans. Currently we know how complex physiological processes regulate circulating testosterone levels, allowing these to fluctuate based on internal and external stimuli on a daily basis. At the molecular level the action of testosterone in tissues, mediated by its binding to androgen receptor, is known to affect several traits related to its anabolic (tissue-building) and androgenic (promotion of male-typical traits) properties. Besides clinical data, animal experiments and anecdotal evidence concerning illegal use of synthetic testosterone derivatives suggest that the hormone has potential to participate into many different biological processes, and that non-physiological testosterone levels can have dramatic effects on some human traits such as muscle build-up and cardiovascular and mental health.
Given this background, it is easy to understand why it is tempting to speculate that individual differences in baseline testosterone levels might explain a substantial proportion of individual variation in risk for many diseases and traits that on average show clear male-female differences. At the same time, however, it is often forgot that linear relationships between normal adult testosterone levels and most traits are however very unlikely. If such relationships would exist, given the 10x difference in testosterone levels between an average man and woman, this should in theory lead to vast differences in the incidence of many complex diseases between the sexes. These two conflicting views sparked us to ask the question that, excluding clinical conditions, to what extent can we actually blame individual differences in baseline testosterone levels for differences in our health and personalities. It turns out that the answer seems more complex than one might expect based on the popular notions.
In our study we took advantage of the fact that testosterone levels are highly heritable: twin studies indicate that the heritability can be up to 65% in males1. In theory, this heritable variation thus might be responsible for a big portion of differences in susceptibility to any common disease showing male-females bias. Through the emergence of genetic and biomarker data from large biobanks, we were well positioned to use such genetic information to explore the causality of testosterone levels to human health.
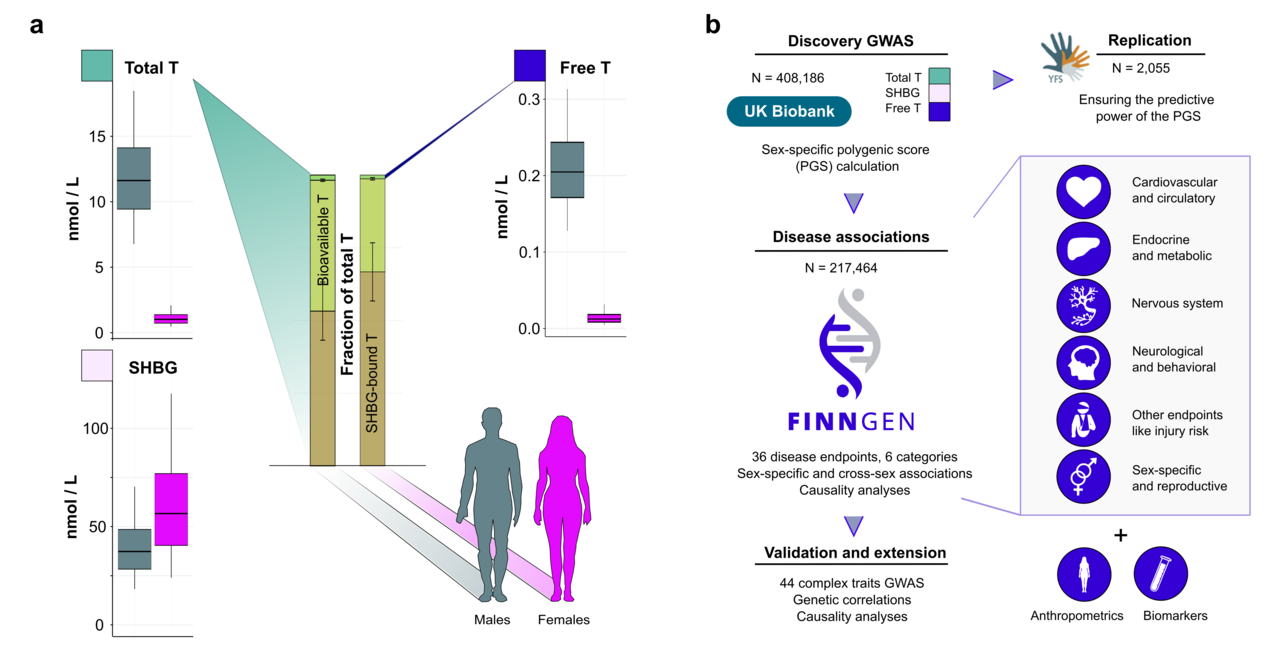
Specifically, in our study we used data from the UK Biobank and FinnGen, the first dataset containing direct testosterone measurements from almost 500,000 British participants 2, and the latter aiming to collect detailed health information from over 500,000 Finns 3,4. The UK biobank data allowed us to construct genetic instruments, polygenic scores (PGS) that we could then use to proxy the consequences of life-long testosterone exposure for the participants of the FinnGen dataset. Combining these associations with a genetic technique called Mendelian Randomization (MR), we could then directly assess the causality of testosterone levels to a wide range of human diseases and traits. In particular, the possibility to assess causality is a clear strength of genetic studies over traditional settings. Additionally, the genetic studies allow for including traits that would be unfeasible or even unethical to study in controlled clinical trials. The main concepts of our study are summarized in Figure 1.
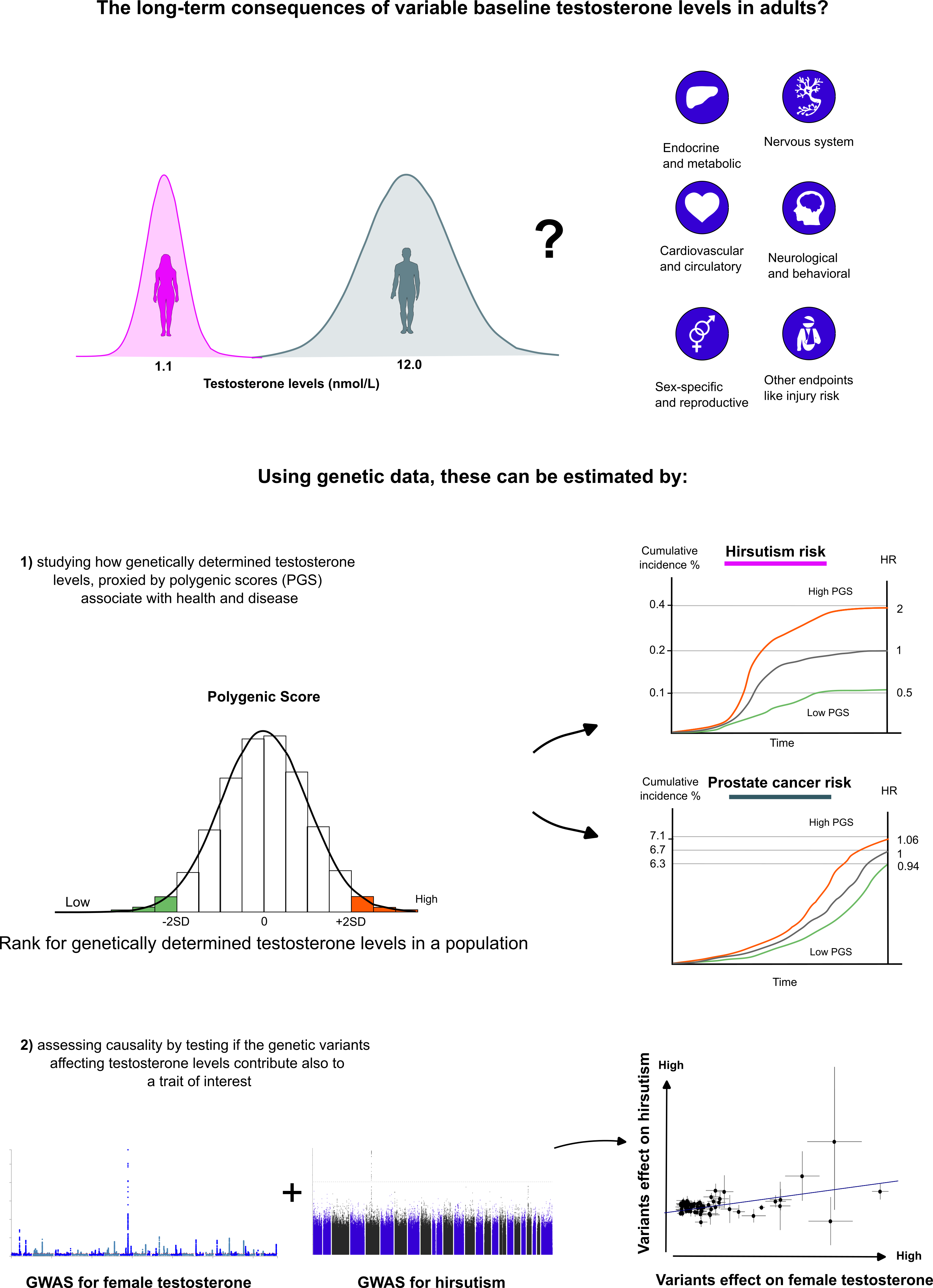
Figure 1. Principles of using genetics to study the relationship between testosterone and health.
The upper panel illustrates the study question, and the panel in the middle shows how the polygenic scores (PGS) can be used to proxy the relationship between testosterone and human traits. The bottom part of the figure introduces the concept of how information from genome-wide association studies (GWAS) can be utilised in determining causality of testosterone to different disease endpoints.
In essence, our study relied on the principle that if the persons carrying a higher number of genetic variants causing higher testosterone levels show higher risk for certain diseases, the disease associations are likely directly attributable to testosterone. This however is partly an oversimplification, as the disease associations may be confounded, e.g., by of so-called genetic pleiotropy, meaning that the genetic variants causing higher or lower testosterone levels have direct effects also on other traits. We discuss this and the other limitations of genetic studies more extensively in our manuscript, including the fact that we can only predict a relatively small proportion of variance in adult testosterone levels with the genetic data.
Our genetic findings however provide a clear picture on how testosterone levels in both sexes are determined by a complex mix of genetic variants and molecular processes affecting testosterone production, secretion, metabolism and excretion. Interestingly, rather than highlighting individual differences in testosterone production, the genetic findings instead seem to emphasize how important liver function and other metabolic factors are for testosterone levels in adults.
Regarding testosterone level’s role in disease and health, we run into some key findings. First, our observations suggest that normal variation in testosterone levels seems to have a relatively minor impact on our lives, in both sexes. In simple terms, this means that for most people, only by knowing you baseline testosterone levels, one usually cannot infer much about one’s health, appearance or behavior. If these are within a normal range, one probably should not be concerned over having either slightly higher or lower testosterone levels than your peers.
Secondly, despite the evidence that testosterone levels are clearly not the sole determining factor over most traits, we plausibly observed that these anyhow showed clear connections to traits that are linked to testosterone action, such as hemoglobin levels, hormonal cancers and hair growth typical for males. Differences in baseline testosterone thus definitely seem to contribute to many diseases and traits, although – importantly – none of the studied traits seemed to depend on testosterone levels alone. Pointing towards potential reasons for maintaining clear sex differences in average testosterone levels, our results suggested that higher testosterone may in general have more beneficial effects for males than for females. Nonetheless, it is worth noting that in both sexes higher testosterone levels were linked with both positive and negative effects on different health measures.
In general, our study supports the idea that maintaining healthy metabolism promotes healthy testosterone levels in both sexes, rather than vice versa. To illustrate this in practical terms, although higher testosterone levels correlate positively with overall health rating in male participants from the UK biobank, (those rating their health as excellent have on average higher levels), based on the genetic data higher testosterone seems not to be the cause for better health, but rather an outcome. For women, such a linear relationship between testosterone and overall health does not seem to exist, but echoing the male data, we similarly detected evidence for metabolism (e.g. triglyceride levels) affecting testosterone in females, instead of the other way around.
Overall, our findings highlight the complex relationship between testosterone and disease risks, as illustrated in Figure 2. It is also good to note that, for example, even though the persons who are genetically predisposed to the highest levels of testosterone seem to be at a slightly higher risk for hormonal cancers, this alone does not explain why an individual gets diagnosed with such a cancer. The same principle appeared clearly applicable to all traits with connections to testosterone, including traits related to athletic capacity, appearance and behavior.
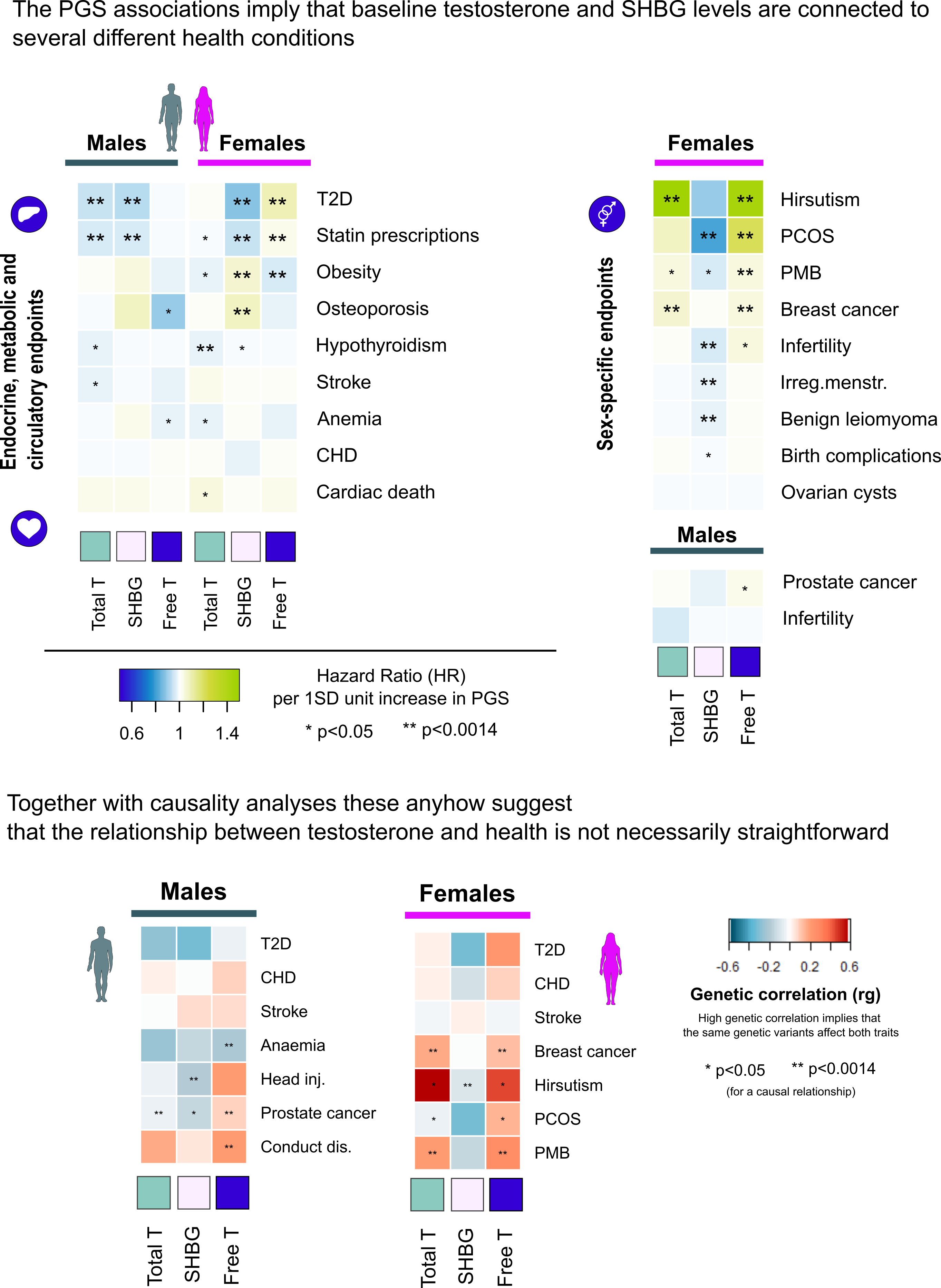
Notably, the genetic estimates seemed to proxy real life data relatively well, as exemplified by the effects on prostate cancer. In our study an increase of one standard deviation (SD) in a polygenic score for free testosterone (explaining only a fraction of variance in free testosterone) increased prostate cancer risk in males by a hazard ratio (HR) of 1.03 (1.02-1.04). The Mendelian Randomisation (MR) estimates from FinnGen in turn suggested a HR of 1.16 (1.09-1.23) per 1SD increase in actual free testosterone. For comparison, a recent observational study using the UK biobank data estimated that an increase of slightly less than 1SD in free testosterone (50pmol/L) amplified prostate cancer risk HR to 1.10 (1.05-1.15)5. It thus seems that genetic methods, despite their limitations, can provide relatively accurate estimates of the magnitude of effects of normal variation in baseline testosterone levels. For those persons with either very low or very high genetic load for testosterone, information of this genetic risk thus might prove clinically useful in the future.
Taken together, our study suggests that whereas at the population level genetically set baseline testosterone levels indeed contribute to many human traits with sex differences, in most cases these alone explain relatively little about human health. Rather than seen as the most important culprit for many human traits, behavior and disease status, it thus seems that normal variation in adult testosterone levels could be better perceived as one complex trait among the others.
References:
1. Harris, J.A., Vernon, P.A. & Boomsma, D.I.J.B.G. The Heritability of Testosterone: A Study of Dutch Adolescent Twins and Their Parents. Behav. Genet. 28, 165-171 (1998).
2. Bycroft, C., et al. The UK Biobank resource with deep phenotyping and genomic data. Nature 562, 203-209 (2018).
3. Kurki, M.I., Karjalainen, J., Palta, P. et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature 613, 508–518 (2023).
4. Heyne, H.O., Karjalainen, J., Karczewski, K.J. et al. Mono- and biallelic variant effects on disease at biobank scale. Nature 613, 519–525 (2023).
5. , , , et al. Circulating insulin-like growth factor-I, total and free testosterone concentrations and prostate cancer risk in 200 000 men in UK Biobank. Int. J. Cancer. 2021; 148: 2274– 2288.
Follow the Topic
-
Communications Medicine

A selective open access journal from Nature Portfolio publishing high-quality research, reviews and commentary across all clinical, translational, and public health research fields.
Related Collections
With Collections, you can get published faster and increase your visibility.
Reproductive Health
Publishing Model: Hybrid
Deadline: Mar 30, 2026
Healthy Aging
Publishing Model: Open Access
Deadline: Jun 01, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in