Unexpected discovery of a novel DMS production enzyme in diverse marine bacteria
Published in Microbiology
This work is based on a long-standing and successful collaboration between Ocean University of China and the University of East Anglia studying the mechanisms that microbes use to metabolize the abundant marine osmolyte dimethylsulfoniopropionate (DMSP) and the climate-active gas dimethylsulfide (DMS). DMSP, made in billion tonne quantities annually, is thought to be the major source of DMS via diverse microbial DMSP lyase enzymes that cleave DMSP to liberate DMS. DMS is best known as a significant contributor to the characteristic smell of the seaside, but it is also an important signaling molecule, nutrient, and when released into the atmosphere, its oxidation products are important cloud condensation nuclei, potentially influencing climate and returning sulfur back to land. Furthermore, DMS has been seen by recent press as a potential indicator of life on distant planets. For these reasons, DMS has attracted widespread attention from researchers, but key questions remain as to the microbes that produce this compound, how and why they do so in diverse environments.
In 2015 and 2023, alternative microbial DMSP-independent routes to DMS production were discovered from the methylation of the abundant sulfur gases methanethiol (MeSH) and hydrogen sulfide (H2S) via an enzyme termed MddA1,2. Although this enzyme does exist in some marine algae, bacteria and haloarchaea, these microbes are not abundant in marine environments. However, microbes with mddA and the potential to produce DMS are highly abundant in terrestrial sediment environments. This led to hypothesis that MeSH and H2S dependent DMS production was significant in terrestrial but not marine environments and that DMSP cleavage was the major route to global DMS production.
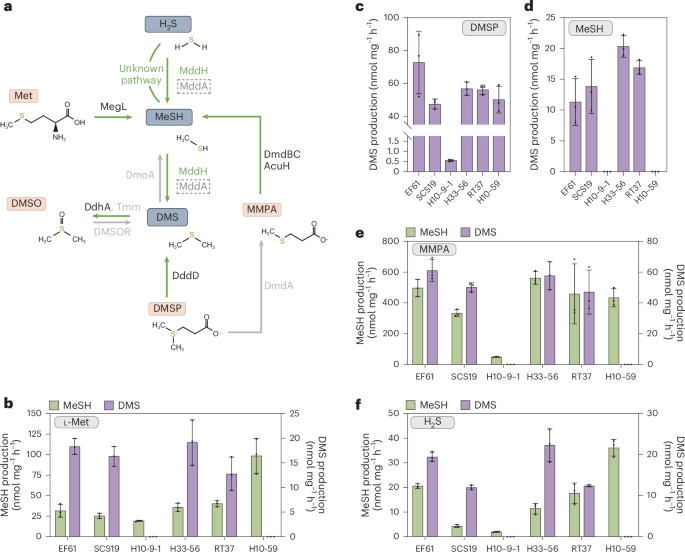
From a previous expedition to the Mariana Trench, we isolated an interesting gammaproteobacterium Halomonas alimentaria EF61 from 3600-meter-deep seawater that altimately impacts the above belief about DMS production. This bacterium excited us because it produced DMS without addition of DMSP, leading us to predict that it could produce and cleave DMSP itself, as was the case with Labrenzia aggregata, in which the first DMSP synthesis genes were identified from3. However, we soon realized that although EF61 could cleave DMSP and contained the DMSP lyase enzyme DddD4, it did not produce this osmolyte. Instead, we showed that EF61 produced DMS from the methylation of H2S and MeSH. More excitedly, we discovered that the EF61 genome lacked MddA, the only known H2S and MeSH S-methyltransferase, and thus must contain a novel Mdd enzyme/s. Our next step was to examine if H2S and MeSH dependent DMS production was common to other Halomonas strains, and here we got lucky as we found that while some strains were able to produce DMS from H2S and MeSH, others were not. This provided us with the perfect situation to use comparative genomic analysis to identify methyltransferases common only to those Halomonas strains producing DMS. Fortunately, of 84 genes common to the DMS producing Halomonas strains, only one encoded a methyltransferase, which was annotated as a ubiquinone methyltransferase UbiE. When this gene was deleted in H. alimentaria EF61, the resultant strain no longer produced DMS from H2S and MeSH. Furthermore, when purified this protein, termed MddH, showed H2S and MeSH S-methylation activity, confirming the function of this new H2S and MeSH S-methyltransferase enzyme.
MddH showed no or very limited protein sequence identity to MddA or the recently identified human thiol methyltransferases, respectively. Furthermore, mddH was widespread and encoded a functional H2S and MeSH S-methyltransferase enzyme in diverse marine bacteria and, excitedly, was also abundant and transcribed in marine metagenomic and metatranscriptomic data. We predicted that approximately 5% and 15% of bacteria in marine seawater and sediment contain mddH, respectively, and thus the potential to produce DMS independently of DMSP. These marine abundance levels are far higher than for mddA, and mddH transcript levels were comparable to the most abundant DMSP lyase gene, dddP. These findings potentially challenge the common belief that DMSP cleavage is the only significant source of DMS in marine settings.
For MddH to have prominent role in marine DMS production its substrates would have to be available in the environment. MeSH is a reactive gas that is rapidly consumed and reported at 0.3-2 nM dissolved levels in seawaters. The genetic potential to produce MeSH from methionine (via megL), DMSP and methylmecaptoproionate (via DMSP demethylation) is hugely abundant in marine bacteria and environments. Furthermore, H2S can reach micromolar-millimolar concentrations in hydrothermal vent and marine sediment environments, where mddA and mddH are known to be more abundant. Therefore, MeSH and H2S could be an important source for DMS production through MddH in certain marine environments, much like MddA could be in terrestrial settings.
It should be noted that research into DMSP cleavage has received far more and diverse attention than H2S/MeSH dependent pathways. To date molecular research, such as done here, has elucidated ten different DMSP lyases (encoding by ddd and Alma genes) in diverse algae, bacteria, corals and fungi, but only two microbial mdd genes for H2S/MeSH dependent DMS production are known. Although most bacteria that we have screened with H2S/MeSH dependent DMS production phenotypes contained either mddH or mddA, there are many exceptions that likely possess novel Mdd enzymes. Furthermore, our research also highlights that the Halomonas strains studied here also have MddH independent routes to S-methylate and potentially detoxify H2S that do not function on MeSH. It is remarkable that there is such biodiversity in the ways in which microbes cleave DMSP and generate DMS from MeSH and H2S.
In conclusion, our research offers novel insights into how marine bacteria generate DMS, leading to a better understanding of the global sulfur cycle and potentially a change in the perception of how DMS is generated in marine environments. It emphasizes the importance of the application of molecular genetics to biogeochemical cycling - there is clearly much biodiversity left to discover.
References
- Carrión, O. et al. A novel pathway producing dimethylsulphide in bacteria is widespread in soil environments. Nat. Commun. 6, 6579 (2015).
- Li, C. Y. et al. Aerobic methylation of hydrogen sulfide to dimethylsulfide in diverse microorganisms and environments. ISME J. 2023 178 17, 1184–1193 (2023).
- Curson, A. R. J. et al. Dimethylsulfoniopropionate biosynthesis in marine bacteria and identification of the key gene in this process. Nat. Microbiol. 2, 17009 (2017).
- Todd, J. D. et al. Structural and regulatory genes required to make the gas dimethyl sulfide in bacteria. Science. (2007).
Follow the Topic
-
Nature Microbiology

An online-only monthly journal interested in all aspects of microorganisms, be it their evolution, physiology and cell biology; their interactions with each other, with a host or with an environment; or their societal significance.
Related Collections
With Collections, you can get published faster and increase your visibility.
The Clinical Microbiome
Publishing Model: Hybrid
Deadline: Mar 11, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in