Unexpected Gender Differences in Progressive Supranuclear Palsy Reveal Efficacy for Davunetide in Women
Published in Biomedical Research

This is my personal point of view, behind the scenes of my article entitled “Unexpected Gender Differences in Progressive Supranuclear Palsy Reveal Efficacy for Davunetide in Women” Gozes et al., Translational Psychiatry, October 16, 2023. Starting from the inception of what I see as my life discovery, at the turn of the century, we revealed a new protein to science, and called it activity-dependent neuroprotective protein (ADNP). We then tried to develop knockout mice, and to our surprise, this mission was incompatible with life, highlighting the critical function of ADNP. Indeed, de novo mutations in ADNP cause the ADNP syndrome and somatic mutations in ADNP parallel Alzheimer’s disease progression. Important to add is that our expression cloning of the mouse ADNP cDNA included detection with antibodies recognizing the Ser-Ile-Pro (SIP) peptide motif, which, in turn, binds microtubule end binding proteins (EB1 and EB3), and enhances binding of other proteins to microtubules, an amplifier effect, essential for dendritic spine formation and axonal transport.
Using a reductionist approach, we synthesized peptides spanning this SIP motif and discovered the small peptide Asp-Ala-Pro-Val-Ser-Ile-Pro-Gln (NAPVSIPQ), which we called NAP, then AL-108, davunetide and CP201. We then revealed that the NAPVSIP motif interacts with SH3 domains also impacting the actin cytoskeleton and association with synapse formation. Lastly, the NAPVSIPQ domain interacts with the armadillo sequence of beta catenin, essential for the critical WNT signaling pathway and with a zinc finger on ADNP critical for transcription. Most importantly, a missense mutation in NAPVSIPQ (i.e., NAPVSIPQQ to NAPVSIPQE, at the protein level) led to the ADNP syndrome (intellectual and motor delays) in an infant. You may ask yourself at this point, but what does this have to do with Tau, sex and progressive supranuclear palsy?
Regarding Tau, EB1/EB3 enhance Tau – microtubule interaction, and NAP (through its SIP motif) dramatically augments this interaction, thus protecting against Tau hyperphosphorylation and Tau aggregation – tauopathy. Coupled with regulation of the Akt pathway through WNT signaling regulation, NAP further inhibits Tau hyperphosphorylation through glucagon synthase 3b (GSK3b). In this respect, ADNP mutations inflict tauopathy, as we also discovered in postmortem tissues of a young child.
Thus, the connection to tauopathy is clear, and while ADNP levels parallel performance in IQ tests, NAP (davunetide) protected cognitive scores in individuals suffering from amnestic mild cognitive impairment.
With this is mind, more than a decade ago, Allon Therapeutics, the company that we founded together with Ramot at Tel Aviv University, in collaboration with businessmen, embarked on a global clinical trial assessing davunetide in progressive supranuclear palsy (PSP), a pure tauopathy. The trial was led by the best neurologists, key opinion leader, who designed it on the assumption that men and women PSP patients have similar disease characteristics and hence will respond to davunetide treatment in the same fashion. Analyzing the results accordingly, the study concluded no efficacy. As such, Ramot requested and received the technology back including all raw data for further development and licensing.
Disappointed and perplexed by the trial results, I personally looked for reasons to explain this, suggesting 1] a possible late start (with advanced neurodegeneration that is not treatable), and/or 2] excessive dose and/or 3] specific disease mechanism compared to AD bearing in mind that PSP shows deposition of a specific Tau isotype including 4 repeated domains of tubulin/microtubule binding, contrasting AD that includes both 3 and 4 repeated tubulin/microtubule binding domains (3R and 4R Tau), with NAP (davunetide) enhancing more favorably the 3R Tau tubulin/microtubule association.
However, the point of sex influence remained as an open question. Considering that ADNP is regulated by the estrous cycle, and in turn ADNP regulates sex steroid biosynthetic enzymes, we thought that perhaps the sex influence should be revisited. Further considering that testosterone regulates Akt/GSK3b and inhibits Tau hyperphosphorylation, with women representing 70% of the most prevalent tauopathy, AD and with the progression of Tau deposition progression correlating with menopause onset age, tauopathy is suggested to be sex dependent.
Thus, in our opinion (now gaining a momentum), we should not treat brain deterioration in men and women identically and mix the tested subjects assuming a similar disease course and equal drug dosing. With this in mind, we have now conducted a new detailed analysis in which men and women were meticulously analyzed separately. In doing so, we found dramatic sex differences and discovered that davunetide showed protective activity (efficacy) in women, as detailed in our article Abstract:
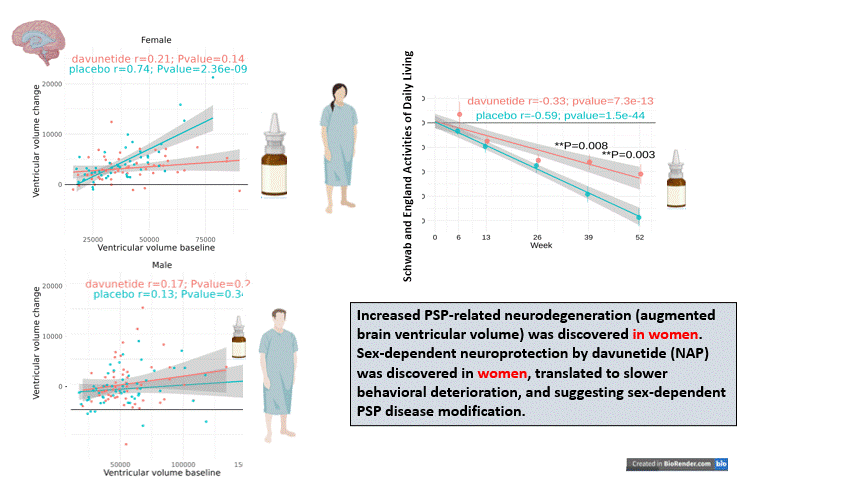
“Analyzing sex-dependency in a 52 week-long- PSP clinical trial (involving over 200 patients) demonstrated clear baseline differences in brain ventricular volumes, a secondary endpoint. Dramatic baseline ventricular volume-dependent/volume increase correlations were observed in 52-week-placebo-treated females (r=0.74, P=2.36-9), whereas davunetide-treated females (like males) revealed no such effects. Thus, the disease seems to progress differently in men and women, furthermore, in general, ventricular volumes and brain size on the who differ between men and women, questioning the idea of mixing those results at the first place.
Indeed, “assessment of primary endpoints, by the PSP Rating Scale (PSPRS) and markedly more so by the Schwab and England Activities of Daily Living (SEADL) scale, showed significantly faster deterioration in females, starting at trial week 13 (P=0.01, and correlating with most other endpoints by week 52). Twice daily davunetide treatments slowed female disease progression and revealed significant protection according to the SEADL scale as early as at 39 weeks (P=0.008), as well as protection of the bulbar and limb motor domains considered by the PSPRS, including speaking and swallowing difficulties caused by brain damage, and deterioration of fine motor skills, respectably (P=0.01), at 52 weeks. Furthermore, at 52 weeks of trial, the exploratory Geriatric Depression scale (GDS) significantly correlated with the SEADL scale deterioration in the female placebo group and demonstrated davunetide-mediated protection of females. Female-specific davunetide-mediated protection of ventricular volume corresponded to clinical efficacy. Together with the significantly slower disease progression seen in men, the results reveal sex-based drug efficacy differences, demonstrating the neuroprotective and disease-modifying impact of davunetide treatment for female PSP patients.”
With PSP still being an unmet medical need and with ExoNavis now holding an exclusive from Ramot, we now see a clear path for davunetide development for women suffering from PSP and beyond.
Follow the Topic
-
Translational Psychiatry

This journal focuses on papers that directly study psychiatric disorders and bring new discovery into clinical practice.
Related Collections
With Collections, you can get published faster and increase your visibility.
Moving towards mechanism, causality and novel therapeutic interventions in translational psychiatry: focus on the microbiome-gut-brain axis
Publishing Model: Open Access
Deadline: May 19, 2026
From mechanism to intervention: translational psychiatry of childhood maltreatment
Publishing Model: Open Access
Deadline: Feb 28, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in