Unleashing the power of DNA: fighting cancer with tailored vaccines
Published in Cancer
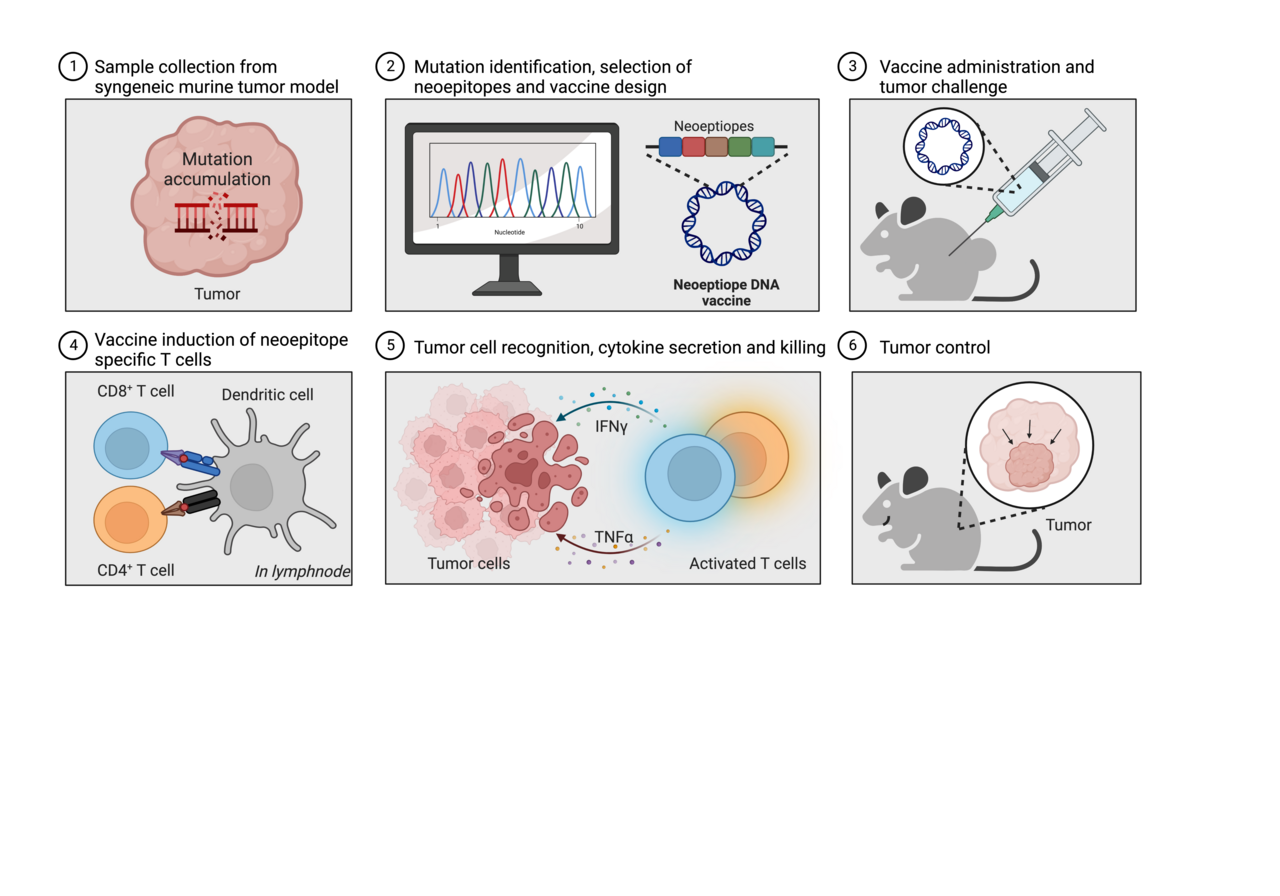
Background
Neoantigens hold great promise as targets for cancer immunotherapy as they are unique protein fragments that arise from tumor-specific genetic mutations, enabling targeted immune responses against cancer cells while sparing normal cells. The antigenic determinants of neoantigens are termed neoepitopes, and they have the potential to be presented directly on the surface of tumor cells by the major histocompatibility complex (MHC) and therefore to be recognized and targeted by T cells.
Several neoantigen vaccine formulations have been and are currently being tested in clinical phase I and II trials, delivering neoantigens as e.g. mRNA, DNA, adjuvanted peptides, and dendritic cells loaded with peptides1–5. These neoantigen vaccine trials have had promising outcomes, displaying good safety profiles and ability to elicit expansion of neoantigen-specific T cells in treated patients. Recently, Moderna and Merck announced positive data from a phase 2 clinical trial obtaining significantly improved relapse free survival of high-risk melanoma patients after treatment with their neoantigen mRNA vaccine in combination with anti-PD-1 (αPD-1) antibody, compared to survival of αPD-1 monotherapy6.
Preclinical studies with murine models of cancer have provided us with the initial proof of concept for the utilization of neoantigen vaccines in cancer therapy and are continuously of translational relevance, providing insights into immune recognition and destruction of cancer cells. These preclinical investigations also utilize varying vaccine formulations (mRNA, DNA, viral vectors, and adjuvanted peptides) with evident ability to elicit tumor-specific immune responses and hamper or prevent tumor growth7–10.
Study objectives
In this study we set out to investigate the effectiveness of plasmid DNA to deliver neoantigens in two mouse models of cancer. This included evaluation of the ability to prevent or delay tumor growth and, furthermore, monitoring of the induction of specific immune cell (i.e. T-cell) reactivity upon neoantigen DNA vaccination.
Major findings reported
Prophylactic vaccination with neoantigen-encoding plasmid DNA can prevent or delay tumor growth in two murine cancer models
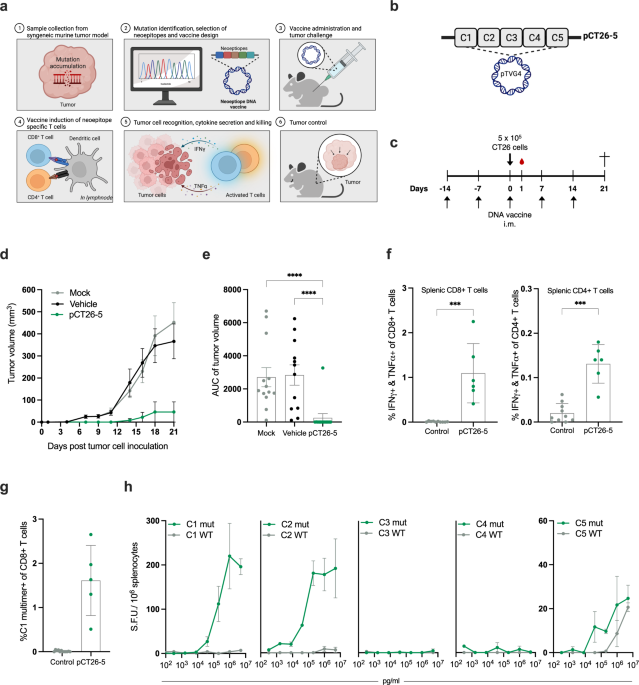
We vaccinated mice weekly with plasmid DNA encoding 5 or 13 in silico-predicted neoepitopes from the CT26 tumor model or empty (“mock”) DNA as a comparator. After two weeks, mice were challenged subcutaneously with CT26 tumor cells and thereafter monitored for tumor growth. Consistency, mice immunized with CT26 neoantigen DNA developed significantly smaller and fewer tumors than the mock group. In addition, we measured significantly higher presence of neoepitope specific CD4+ and CD8+ T cells in blood and splenocytes of CT26 neoantigen DNA immunized mice compared to the mock group. The potency and longevity of the immune response resulting from our DNA vaccine platform was apparent, as a single dose of the neoantigen vaccine could prevent tumor development in ∼40% of the mice when challenged with CT26 tumor cells more than 200 days after the single DNA vaccination.
Our observations were further validated in the rapidly growing B16F10 mouse cancer model. Here, we were able to confirm that immunization with plasmid DNA encoding 13 in silico-predicted neoepitopes from B16F10 led to significantly lower tumor volume compared to the mock DNA group.
Both the CD4+ and CD8+ T cell subsets contribute to anti-tumor responses
To understand the immune response associated with tumor rejection, we selectively depleted CD4+ or CD8+ T cells in mice immunized with neoantigen encoding DNA. We found that depletion of CD8+ T cells completely abrogated the ability to prevent tumor growth, emphasizing their crucial role in mediating a tumoricidal immune response. Depletion of CD4+ T cells had a less detrimental effect, suggesting their involvement primarily in shaping and improving CD8+ T-cell responses, rather than directly interacting with the tumor cells.
Combination therapy with immune checkpoint inhibition provided an additive effect
Next, we investigated the potential of combining our neoantigen DNA vaccine with immune checkpoint inhibitors as they are the current standard of care for several solid cancer indications in the clinic. We combined a sub-optimal dose of our neoantigen DNA vaccine (i.e. a low, non-protective dose) and αPD-1 checkpoint inhibitor treatment, before and during tumor challenge with CT26 cells. Our results showed an additive effect of the neoantigen DNA and αPD-1 immunotherapy, reducing tumor burden and prolonging survival of the treated mice, compared to mice treated with either monotherapy on their own. The benefit of the combination therapy was also apparent from analyses of the accompanying immune responses, leading to superior induction of neoepitope specific T cells in spleens and tumors of the mice.
Closing remarks
We are excited to report that multi-neoantigen plasmid DNA vaccination resulted in anti-tumor immune responses across two murine cancer models. These responses included long-lasting neoepitope-specific T-cell responses in the blood, spleen, and tumors after immunization. We also demonstrated that engaging both the CD4+ and CD8+ T cell compartments was crucial for suppressing tumor growth and obtaining broad and high frequent T-cell responses. Furthermore, combining low-dose DNA vaccination with immune checkpoint inhibitor αPD-1 showed an additive effect that surpassed the effectiveness of either therapy alone.
Our findings highlight the versatility of DNA vaccination as a platform for encoding multiple neoantigens into a single vaccine entity, making it a feasible strategy for personalized neoantigen immunotherapy through vaccination. The here described neoantigen DNA vaccine represents a murine surrogate for our clinical compound, EVX-02. EVX-02 has recently been tested in a clinical phase 1/2a trial (NCT04455503), in which fully resected melanoma patients were treated with αPD-1 (Nivolumab) and patient-tailored DNA plasmids encoding 13 in silico-predicted neoepitopes as adjuvant immunotherapy. The 10 patients who received the full dosing schedule were relapse-free at their last assessment and the combination therapy of Nivolumab and EVX-02 was well tolerated in patients, leading to induction of long-lasting neoepitope specific T-cell responses11. It remains costly and time-consuming to produce patient-tailored vaccines, but we believe that our findings, along with those of other key players such as Moderna and Merck, confirms the rationale and huge potential of using neoantigen-based vaccines for cancer immunotherapy.
References
- Sahin, U. et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 547, 222–226 (2017).
- Ott, P. A. et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 547, 217–221 (2017).
- Carreno, B. M. et al. A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells. Science (80-. ). 348, 803–808 (2015).
- Yarchoan, M. et al. 453 Personalized DNA neoantigen vaccine (GNOS-PV02) in combination with plasmid IL-12 and pembrolizumab for the treatment of patients with advanced hepatocellular carcinoma. J. Immunother. Cancer 9, A481 LP-A481 (2021).
- Krauss, J. et al. Abstract CT274: Individualized APC targeting VB10.NEO cancer vaccines induce broad neoepitope-specific CD8 T cell responses in patients with advanced or metastatic solid tumors: interim results from a phase 1/2a trial. Cancer Res. 83, CT274–CT274 (2023).
- Moderna. mRNA-4157 (V940) in combination with KEYTRUDA reduced the risk of recurrence or death by 44% compared to KEYTRUDA alone in stage III/IV melanoma patients with high risk of recurrence following complete resection. (2023). Available at: https://news.modernatx.com/news/news-details/2023/Moderna-and-Merck-Announce-mRNA-4157-V940-an-Investigational-Individualized-Neoantigen-Therapy-in-Combination-with-KEYTRUDAR-Pembrolizumab-Demonstrated-Superior-Recurrence-Free-Survival-in-Patients-with-High-Risk-Stage-IIIIV-Melanoma-Following-Comple/default.aspx. (Accessed: 1st June 2023)
- Kreiter, S. et al. Mutant MHC class II epitopes drive therapeutic immune responses to cancer. Nature 520, 692–696 (2015).
- Tondini, E. et al. A poly-neoantigen DNA vaccine synergizes with PD-1 blockade to induce T cell-mediated tumor control. Oncoimmunology 8, 1652539 (2019).
- D’Alise, A. M. et al. Adenoviral vaccine targeting multiple neoantigens as strategy to eradicate large tumors combined with checkpoint blockade. Nat. Commun. 10, 1–12 (2019).
- Castle, J. C. et al. Exploiting the mutanome for tumor vaccination. Cancer Res. 72, 1081–1091 (2012).
- Kleine-Kohlbrecher, D. et al. Abstract LB199: A personalized neoantigen vaccine is well tolerated and induces specific T-cell immune response in patients with resected melanoma. Cancer Res. 83, LB199–LB199 (2023).
Follow the Topic
-
npj Vaccines

A multidisciplinary journal that is dedicated to publishing the finest and high-quality research and development on human and veterinary vaccines.
Related Collections
With Collections, you can get published faster and increase your visibility.
Therapeutic HPV vaccines
Publishing Model: Open Access
Deadline: Jun 30, 2026
Lipid nanoparticle (LNP)-adjuvanted vaccines
Publishing Model: Open Access
Deadline: May 19, 2026





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in