Unlocking the potential of allogeneic Vδ2 T cells for ovarian cancer therapy through CD16 biomarker selection and CAR/IL-15 engineering
Published in Cancer
γδ T cells, constituting approximately 1% to 5% of total human peripheral T cells, are a crucial subset of T cells. Of these, approximately 80% to 85% express the TCR Vδ2 chain. Because the TCR Vδ2 chain is almost exclusively paired with the TCR Vγ9 chain, they are also known as Vγ9Vδ2 T cells1. Prior clinical trials employing Vδ2 T cells for adoptive transfer therapy, whether in autologous or allogeneic contexts, produced varied results. While certain cancer patients exhibited favorable therapeutic outcomes following this treatment, others showed no response. The incongruent findings underscore the necessity of identifying biomarkers capable of predicting responses to immunotherapy involving Vδ2 T cells2.
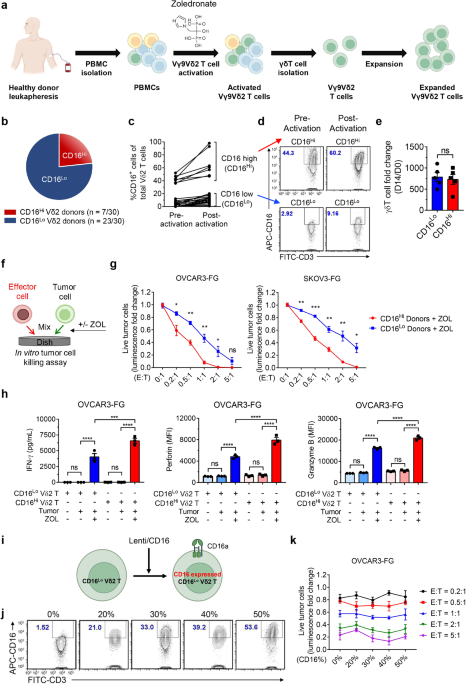
Our objective was to develop CAR-Vδ2 T cell-based immunotherapy. Preceding CAR engineering, a series of killing assays were conducted using Vδ2 T cells sourced from diverse peripheral blood mononuclear cell (PBMC) donors and employed against various cancer cell lines. Striking variations in the tumor-killing abilities of Vδ2 T cells among donors prompted further investigation. Flow cytometry analysis revealed that donors with better performance exhibited a higher prevalence of CD16+ Vδ2 T cells, and their Vδ2 T cells demonstrated elevated CD16 expression, categorizing them into the CD16Hi group. CD16 (FcγRIII), widely acknowledged as an IgG receptor facilitating antibody-dependent cell-mediated cytotoxicity (ADCC), is important for antitumor activities of many therapeutic antibodies. While the majority of CD16 research has centered on NK cells, some studies have reported CD16 expression by Vδ2 T cells3. Notably, Vδ2 T cells from the majority of donors expressed low CD16, forming the CD16Lo group, distinguishing them from NK cells.
When co-cultured with the ovarian cancer cell line OVCAR3 in the presence of Zoledronate (ZOL), an FDA-approved small molecule compound known to activate Vδ2 T cells4, CD16Hi Vδ2 T cells exhibited elevated secretion of cytotoxic molecules, including perforin and granzyme B, as well as increased IFN-γ levels compared to CD16Lo Vδ2 T cells. This robust effector function may elucidate the superior tumor-killing capacity of CD16Hi Vδ2 T cells over CD16Lo counterparts. Notably, the overexpression of transgenic CD16 on CD16Lo Vδ2 T cells by lentiviral vectors did not augment their cytotoxic potential. This observation implies that CD16 could serve as a valuable biomarker for identifying donors with highly potent Vδ2 T cells, rather than functioning as an active receptor directly contributing to tumor killing. Furthermore, the genetic introduction of CD16 to CD16Lo Vδ2 T cells might not reproduce the potent antitumor activity observed in Vδ2 T cells expanded from CD16Hi donors.
Bulk RNA-Sequencing analysis further supported our in vitro assay results. CD16 expression positively correlated with immune effector and activation signatures, including cytotoxicity, degranulation, and innate immunity functions. Furthermore, the CD16Hi group demonstrated a downregulation of RORC, a transcription factor associated with a Th17-like phenotype of γδ T cells. Studies have shown that Th17-like γδ T cells can contribute to the progression of cancer in various syngeneic models, and there is supporting evidence for their adverse effects in human malignancies5.
Encouraged by these findings, we then engineered CD16Hi Vδ2 T cells with mesothelin-targeted CAR (MCAR) and soluble IL-15 using lentiviral vectors. Mesothelin, a cell-surface glycoprotein with minimal expression in normal tissue, exhibits overexpression in numerous solid tumors, including ovarian cancer6. Overexpression of transgenic soluble IL-15 has been shown to enhance the persistence of immune cells in vivo by many groups7,8. In vitro analyses demonstrated the multi-faceted tumor-targeting capabilities of the engineered Vδ2 T cells, encompassing CAR-mediated recognition, ZOL-induced activation of TCRs, and ADCC facilitated by CD16 in the presence of anti-HER2 therapeutic antibody, trastuzumab. This capacity for multi-targeting is crucial, especially given that tumor cells frequently downregulate the expression of their antigens. CAR-engineered CD16Hi Vδ2 T cells may present a promising strategy to counteract situations of tumor antigen escape.
Based on the data demonstrating antitumor efficacy, we proceeded to assess the therapeutic cells in two ovarian cancer xenograft mouse models. Our results revealed that CD16Hi Vδ2 T cells, engineered with MCAR and IL-15, exhibited not only superior antitumor efficacy but also sustained persistence in various mouse tissues, including ovarian tumors. Furthermore, the persistence of engineered Vδ2 T cells did not correlate with xenoreactivity. Histological examination of H&E-stained tissue sections from experimental mice indicated no tissue damage, suggesting that these therapeutic cells are unlikely to induce graft-versus-host disease (GvHD) in patients in allogeneic settings.
In response to current challenges posed by conventional CAR-based αβ T cell therapies, which exhibit limited efficacy against solid tumors, active exploration is underway to employ CAR-engineered innate immune cells, such as Vδ2 T cells. Vδ2 T cells exhibit characteristics from both the adaptive and innate immune systems, offering potential advantages over αβ T cells. These advantages include the ability to target tumors through multiple mechanisms, modulate the tumor microenvironment9, and recruit other immune cells10. Nevertheless, donor-to-donor variations exist, with Vδ2 T cells from certain donors exhibiting superior antitumor functions compared to others. Our investigations suggest that CD16 has the potential to serve as a biomarker for the identification and selection of Vδ2 T cell donors.
We acknowledge that several questions could be addressed in future studies. CD16+ and CD16- Vδ2 T cells could be isolated from CD16Hi Vδ2 T cell donors to facilitate a more comprehensive comparison of their gene expressions, phenotype, and functionality. It would be intriguing to further explore why some donors exhibit a higher frequency of CD16+ Vδ2 T cells and why their CD16 expression is elevated. Additionally, conducting further analysis of CD16Hi Vδ2 T cells using single-cell RNA sequencing and epigenomic sequencing may unveil their etiology and contribute to the development of more refined CD16Hi Vδ2 T cell products.
References
- Lee, D. et al. Human γδ T Cell Subsets and Their Clinical Applications for Cancer Immunotherapy. Cancers. 14, 12 (2022).
- Xu, Y. et al. Allogeneic Vγ9Vδ2 T-cell immunotherapy exhibits promising clinical safety and prolongs the survival of patients with late-stage lung or liver cancer. Cell Mol Immunol. 18, 427– 439 (2021).
- Tokuyama, H. et al. Vγ9Vδ2 T cell cytotoxicity against tumor cells is enhanced by monoclonal antibody drugs - Rituximab and trastuzumab. Int J Cancer. 122, 2526–2534 (2008).
- Meraviglia, S., et al., In vivo manipulation of Vgamma9Vdelta2 T cells with zoledronate and low-dose interleukin-2 for immunotherapy of advanced breast cancer patients. Clin Exp Immunol. 161(2): p. 290-7 (2010).
- Agerholm, R. & Bekiaris, V. Evolved to protect, designed to destroy: IL-17-producing γδ T cells in infection, inflammation, and cancer. Eur J Immunol. 51, 2164–2177 (2021).
- Morello, A., Sadelain, M. & Adusumilli, P. S. Mesothelin-Targeted CARs: Driving T Cells to Solid Tumors. Cancer Discov. 6, 133–146 (2016).
- Liu, E. et al. Cord blood NK cells engineered to express IL-15 and a CD19-targeted CAR show long-term persistence and potent antitumor activity. Leukemia 32, 520–531 (2018).
- Makkouk, A. et al. Off-the-shelf Vδ1 gamma delta T cells engineered with glypican-3 (GPC-3)-specific chimeric antigen receptor (CAR) and soluble IL-15 display robust antitumor efficacy against hepatocellular carcinoma. J Immunother Cancer. 9, 12 (2021).
- Fowler, D.W., et al., Zoledronic acid renders human M1 and M2 macrophages susceptible to Vdelta2(+) gammadelta T cell cytotoxicity in a perforin-dependent manner. Cancer Immunol Immunother. 66(9): p. 1205-1215 (2017).
- van Beek, J.J., et al., Dendritic cell cross talk with innate and innate-like effector cells in antitumor immunity: implications for DC vaccination. Crit Rev Immunol. 34(6): p. 517-36 (2014).
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in