Unravelling the mystery of elephant mortalities in Zimbabwe to find an unexpected culprit – a close relative of Pasteurella multocida
Published in Microbiology

As winter retreated in August 2020, elephants in north-western Zimbabwe began to die. Their tusks were intact and there was no sign that they had been poached or poisoned. Elephant deaths due to anthrax at this time of year aren’t unusual in the region, so that was the thought at first. Veterinarians don’t open the carcass when anthrax is suspected to avoid contaminating the environment with anthrax spores. Instead, we take samples such as blood smears, then burn or bury carcasses if possible. When the first elephant died, it looked like anthrax. Minimal samples were taken that afternoon and the carcass was burned.

Then came a call that two more elephant carcasses had been found. It was too late in the day to get out to them, but the next morning, minimal samples were taken from the two new carcasses. Back at the laboratory, blood smears from all three elephants were examined under the microscope.
None of them had anthrax.
With anthrax ruled out, it was worth investigating further by revisiting the remaining carcasses to collect more samples. The two unburned carcasses were opened, and in came another call; now there was another elephant carcass to visit. The carcasses had enlarged livers and spleens, with numerous haemorrhages across their organs.
The phone rang again the next day. Now there were five new dead elephants – all in the same general area, all seeming to have died at the same time as those from the day before. Conducting a post-mortem on an elephant in the field is a messy, time-consuming, and physically demanding process, even more so in hot weather. Getting to carcasses often requires walking in through difficult terrain, where wild and potentially aggressive animals are free roaming, all while carrying the large amount of kit needed to do the post-mortem. With so many carcasses to visit and the state of decomposition from the heat, only a few samples could be taken.
More elephant carcasses were found over the next few weeks, and there was evidence of inflammation and associated bacterial colonies in the tissues of those that could be sampled. The sudden deaths, pathological findings, and presence of certain bacteria (bipolar-staining coccobacilli) suggested that the elephants had died of haemorrhagic septicaemia. This disease, caused by the bacterium Pasteurella multocida, is seen most commonly in cattle and water buffalo. P. multocida is often found in the mouths of healthy animals but can become pathogenic under certain circumstances – like when it killed around 200,000 endangered saiga antelope in Kazakhstan in 2015.
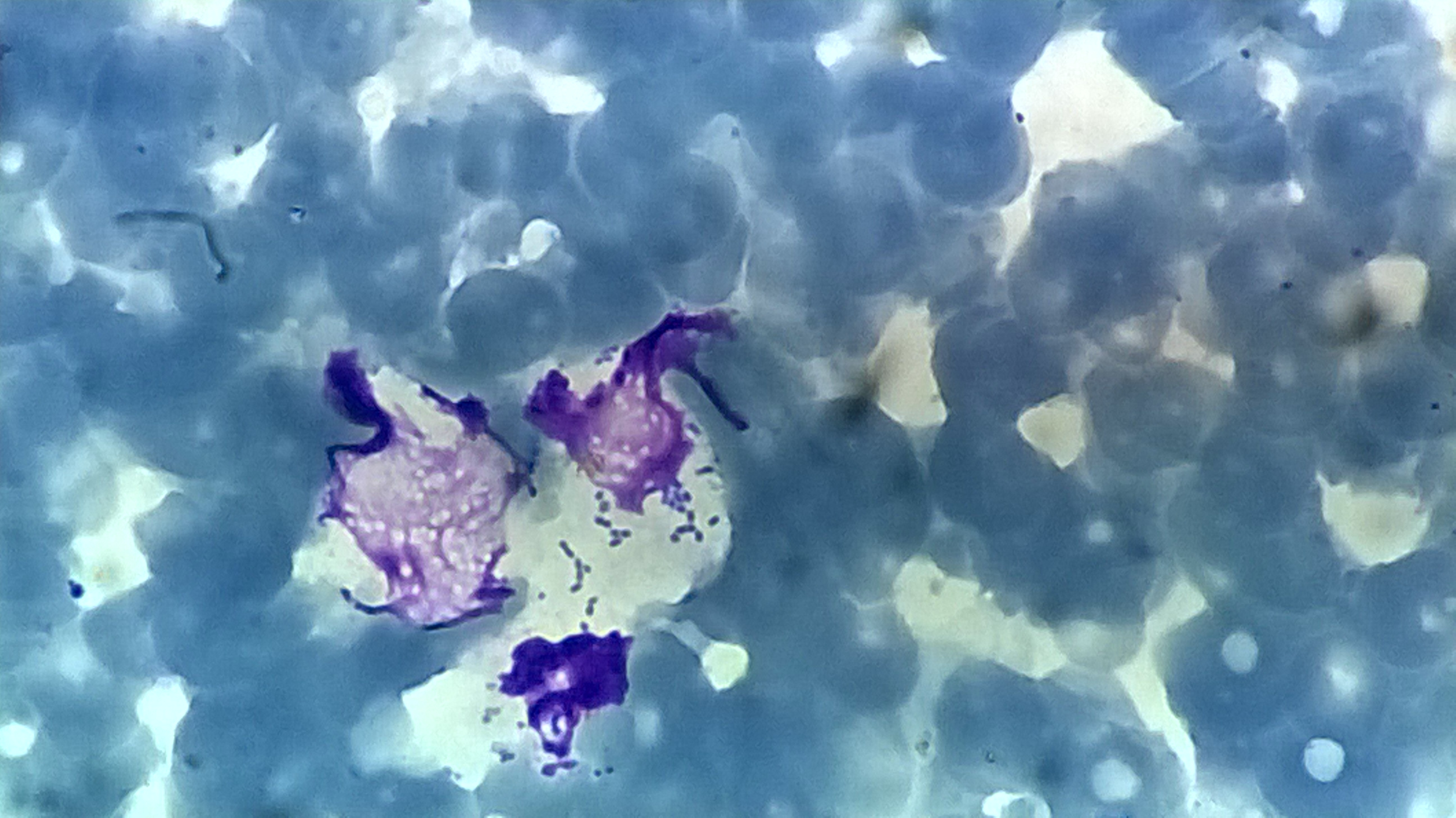
Haemorrhagic septicaemia hadn’t been reported in African elephants before but has been recognized in Asian elephants. A paper from 1906 describes how sudden deaths in domestic Asian elephants in Burma (now Myanmar) were typically attributed to either snakebite or anthrax, until one veterinarian looked in a microscope and saw bipolar-staining coccobacilli like those observed in cases of haemorrhagic septicaemia in cattle. Over 110 years later, the same coccobacilli could be seen in our African elephant samples.
We performed a variety of laboratory tests and found no evidence that other bacteria, viruses, or toxins were underlying the pathology we observed, and started writing up a draft manuscript of our findings on haemorrhagic septicaemia caused by P. multocida. Things took an unexpected turn when isolates from our samples sent to South Africa for culture appeared to be P. multocida but failed to react with any of the four capsular types for this bacterium. The samples were sucrose-negative on further biochemical tests. Together, these results indicated that the isolates were not typical P. multocida after all. Sequencing confirmed that the isolate from the elephant samples was in fact Bisgaard taxon 45 – a very close relative of P. multocida that is yet to be officially named.
Previously reported strains of Bisgaard taxon 45 came from humans who had been bitten by big cats and from healthy psittacines (parrots and their relatives) but had not been associated with septicaemia mortalities like those observed in the elephants in 2020. Whole genome sequencing in our study revealed that Bisgaard taxon 45 shares some of the same virulence factors - molecules that help bacteria infect their hosts and cause disease – with P. multocida. These results led us to completely rewrite our manuscript with Bisgaard taxon 45, not P. multocida, as the culprit.
There are still many questions to be answered about the epidemiology of Bisgaard taxon 45, particularly what triggered this mortality event in 2020. We suggest that a combination of stress from heat and limited forage and water in a high-density population of elephants may have been the underlying cause. This year we could see more elephant deaths - the rains stopped early, winter was relatively warm, and we are now in the hot dry season that is most stressful for wildlife.
What do our findings mean for elephants in the region? The area of Zimbabwe where the mortalities occurred is part of the Kavango-Zambezi (KAZA) Transfrontier Conservation Area, which encompasses over 500,000 km2 and is home to the largest population of elephants in Africa. This area is critical for the conservation of elephants, which are now endangered. In 2022, a group of researchers conducted a large-scale series of aerial surveys to estimate the number of elephants in KAZA. The results of the KAZA Elephant Survey were just released in August: the population size appeared to be stable, but a high number of recent elephant carcasses were observed during the surveys. We don’t know whether Bisgaard taxon 45 caused some of those mortalities, but it should be on the differential diagnosis list for sudden death in African elephants. Further research will be essential to understanding this organism and its relationship with elephants and other animals in the wild, as well as what threat it may pose to wildlife conservation.

Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in