Unveiling the Intricate Mechanism of HD5-Mediated Shigella Invasion: A Scientific Odyssey
Published in Microbiology, Cell & Molecular Biology, and Immunology

The Paradox of HD5: A Host Defense Peptide with Dual Role
Human enteric α-defensin 5 (HD5), an antimicrobial peptide secreted by Paneth cells, is a key component of the innate immune system. While HD5 is known for its pathogen-killing properties, emerging evidence suggests that it paradoxically enhances the infection of certain pathogens, including Shigella and HIV. The absence of a well-defined receptor mediating HD5’s cellular effects has obscured our understanding of its functional complexity. This paradox not only challenges traditional views of host defense peptides but also has significant implications for developing novel therapeutic strategies against infectious diseases.
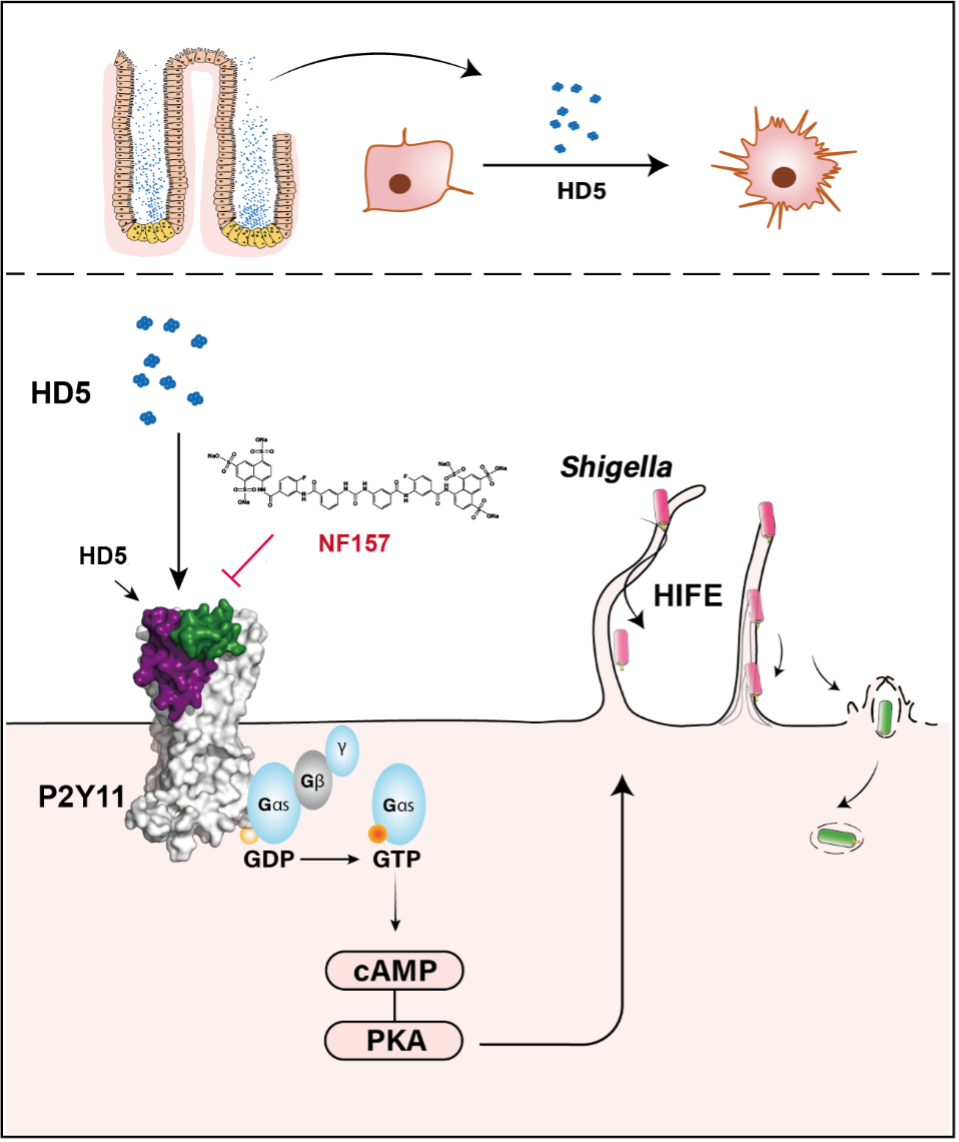 Figure 1. Human enteric α-defensin 5, secreted from the Paneth cell at the base of intestinal crypt, exhibits potent cytoskeleton-regulating activity, inducing filopodia-like extensions (HD5-induced filopodial extensions, HIFE). Shigella, a human-selective enteropathogen, exploits these structures to enhance bacterial adhesion and invasion. Detailed investigation identified P2Y11, a purinergic receptor expressed on human colonic epithelium, as the primary receptor mediating HD5-induced HIFE via Gs-cAMP-PKA signaling pathway. Pharmacological inhibition of P2Y11 using NF157 effectively blocked HD5-mediated Shigella infection, highlighting its potential as a therapeutic target.
Figure 1. Human enteric α-defensin 5, secreted from the Paneth cell at the base of intestinal crypt, exhibits potent cytoskeleton-regulating activity, inducing filopodia-like extensions (HD5-induced filopodial extensions, HIFE). Shigella, a human-selective enteropathogen, exploits these structures to enhance bacterial adhesion and invasion. Detailed investigation identified P2Y11, a purinergic receptor expressed on human colonic epithelium, as the primary receptor mediating HD5-induced HIFE via Gs-cAMP-PKA signaling pathway. Pharmacological inhibition of P2Y11 using NF157 effectively blocked HD5-mediated Shigella infection, highlighting its potential as a therapeutic target.
Discovery of HIFE: A Window into an Unexpected Mechanism
Our investigation began with in vitro Shigella infection assays. Surprisingly, physiological concentrations of HD5 induced rapid and dramatic cytoskeletal rearrangements in HeLa cells, forming abundant filopodial extensions. We termed these structures "HD5-induced filopodial extensions" (HIFE). Live-cell imaging revealed that Shigella actively exploited HIFE for enhanced host-call contact and invasion. Notably, HD5 stimulation increased both the length and density of these filopodial extensions upon HD5 stimulation, underscoring their dynamic nature and role in early-stage infection.
Identifying the HD5 Receptor: A Scientific Pursuit
To uncover the molecular mechanism driving HIFE formation, we systematically ruled out intracellular effects of HD5 and known Shigella-associated receptors. Inspired by previous links between defensins and G-protein-coupled receptors (GPCRs), we hypothesized GPCR involvement. The broad-spectrum GPCR inhibitor suramin significantly suppressed HIFE formation, reinforcing this hypothesis. Through phospho-kinase antibody arrays and global phosphoproteomic analysis, we identified the Gs-cAMP-PKA signaling pathway as the central mechanism through which HD5 induces HIFE. Further transcriptomic and LC-MS/MS analysis pinpointed P2Y11, a purinergic GPCR, as the key receptor mediating HD5-induced cytoskeletal changes. This was validated using the P2Y11-specific inhibitor NF157 and CRISPR/Cas9-mediated P2Y11 knockout, both of which effectively blocked HIFE formation and reduced Shigella invasion.
Deciphering the HD5-P2Y11 Interaction: Structural and Functional Insights
To gain a deeper molecular understanding, we conducted molecular dynamics (MD) simulations to predict key residues involved in the HD5-P2Y11 interaction. Mutational analysis via alanine scanning confirmed the importance of residues R6, T7, E14, R25, L26, and Y27 in HD5-mediated activation of P2Y11. Additionally, we demonstrated that HD5’s native tertiary conformation and dimerization were essential for its functional activity. These findings provide a detailed molecular blueprint of the HD5-P2Y11 interaction and its role in cytoskeletal regulation.
Physiological Relevance: From Gut-on-Chip to In Vivo Studies
To explore the physiological significance of our findings, we utilized multiple experimental models: Gut-on-chip studies confirmed that P2Y11 is apically expressed on the luminal surface of colonic epithelial cells, positioning it for direct interaction with HD5. Human colonic tissue analysis revealed differential P2Y11 expression along intestinal crypts, further supporting the plausibility of HD5-P2Y11 signaling in vivo. Organoid, dynamic gut-on-chip, and guinea pig infection models demonstrated that P2Y11 inhibition (via NF157) significantly reduced HD5-mediated Shigella invasion, highlighting the translational potential of targeting this pathway for therapeutic intervention.
Implications and Future Directions
Our study reveals a novel mechanism by which HD5 enhances Shigella invasion through P2Y11-mediated cytoskeletal remodeling, challenging the conventional view of host defense peptides as strictly antimicrobial agents. This discovery opens exciting new avenues for therapeutic intervention by targeting HD5-P2Y11 interactions to prevent infection. Several key questions emerge from our findings:
- What are the broader physiological functions of the HD5-P2Y11 signaling axis beyond Shigella infection?
- How has Shigella evolved to exploit HD5-induced cytoskeletal changes?
- Could these insights enhance our understanding of host selectivity in human-specific pathogens?
By addressing these questions, future research may further unravel the intricate evolutionary arms race between host defense mechanisms and microbial adaptation, shedding light on novel strategies for combating infection.
Follow the Topic
-
Nature Microbiology

An online-only monthly journal interested in all aspects of microorganisms, be it their evolution, physiology and cell biology; their interactions with each other, with a host or with an environment; or their societal significance.
Ask the Editor - Immunology, Pathogenesis, Inflammation and Innate Immunity
Got a question for the editor about the complement system in health and disease? Ask it here!
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
The Clinical Microbiome
Publishing Model: Hybrid
Deadline: Mar 11, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in