Upcycling of dynamic thiourea thermoset polymers by intrinsic chemical strengthening
Published in Materials
Thermoset polymers are dispensable in our daily life, such as tires, sealants, adhesives and medical devices. However, the excessive use of thermosets has led to exacerbation of plastic pollution, since thermosets are traditionally hard to recycle, giving their crosslinked nature. Tremendous efforts have been made to mitigate the environmental effects of thermosets. Among them, one of the most attractive methods is to make thermosets reprocessible by introducing dynamic bonds into the network or activating dynamic characteristics of otherwise conventional covalent bonds. Upon outside stimulus such as light or heat, dynamic bonds in the polymer network are able to exchange with each other, through which the wasted thermosets could be reprocessed into new shapes and reused. Despite the presented appealing concept, the industrial scale of thermoset reprocessing is still limited. One of the restricts is that the reprocessing of thermosets is inevitably accompanied by mechanical deterioration, lowering the economic value of the recycled materials. The deterioration results from multiple side reactions, such as chain scission, degradation, hydrolyzation and oxidation.
To overcome the influence of side reactions, the most intuitive method is to suppress the occurrence of these reactions. Many traditional methods have already put efforts in this direction such as adding antioxidant or dehydration during reprocessing. However, in such ways, the mechanical deterioration could only be alleviated but never be eliminated due to the complexity of side reactions. On the contrary, by taking a typical example of newly found dynamic thiourea bonds, the work has pointed a new direction to solve the dilemma between thermosets reprocessing and properties worsening. That is, during the reprocessing, the exchange reaction of thiourea bonds enable the materials to be reshaped. More importantly, hindered thiourea bonds embedded in the network can transformed into corresponding urea bonds, offering the materials with stronger hydrogen bonding to enhance mechanical performance. This is a more proactive method compared with suppressing side reactions, since the strengthening of hydrogen bonding gives an opportunity to counteract all the negative consequences from complicated side reactions. In our case, the mechanical decline could be fully avoided, or even the upcycling of thermosets polymer is achieved.
The material synthesis has been illustrated in Fig. 1. After condensation reaction of thiocarbonyldiimidazole and amine, a network with hindered and unhindered thiourea bonds was formed. During reprocessing, both hindered and unhindered thiourea could exchange, enabling reprocessing. More importantly, hindered thiourea can transform into urea bonds, resulting in mechanical strengthening.
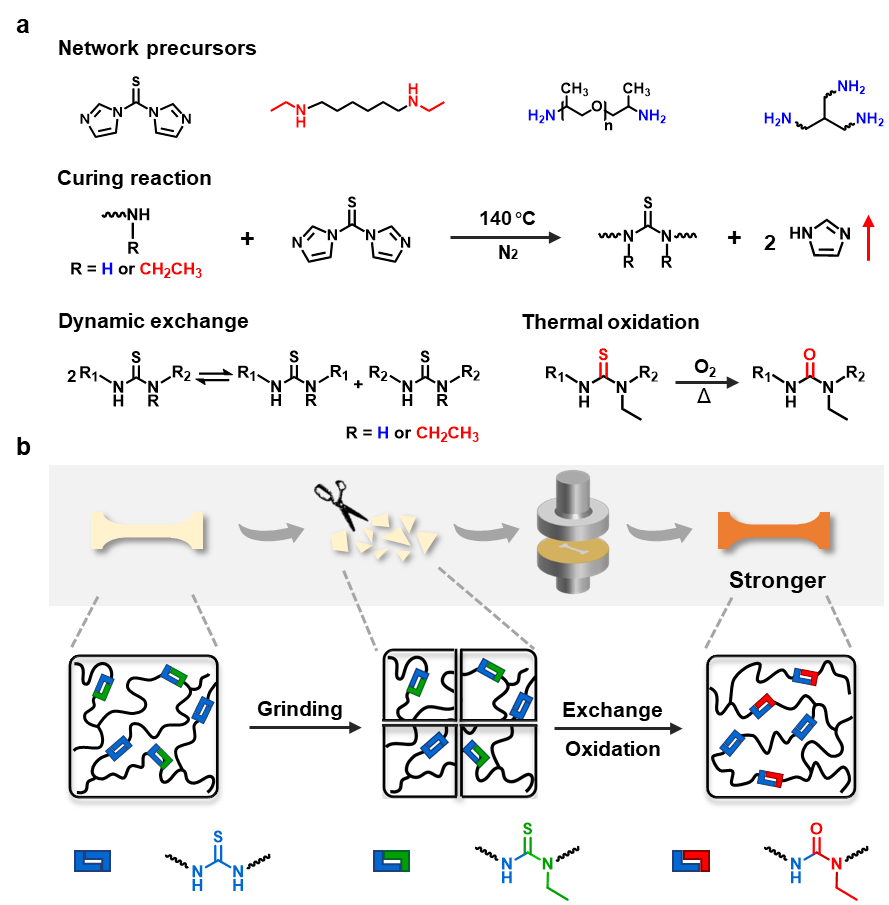
Fig. 1 | Polymer network design and the principle for strengthening. a, The chemistry for network synthesis, reprocessing, and oxidation. b, Schematic illustration of reprocessing and strengthening of the crosslinked network.
The dynamic nature of thiourea bonds has been proven the by small model experiments, with an activation energy of 69.1 kJ/mole (Fig. 2a, Fig. 2b). The oxidation behavior of hindered thiourea bonds has been investigated both through Fourier-transform infrared spectroscopy (FTIR) of polymer films and small molecular experiments (see our published work). While remained almost unchanged in FTIR after thermal treatment in nitrogen at 140 °C for 48 hours (Fig. 2c), the films did show a peak corresponding to urea band in FTIR after heated at 140 °C for 48 hours in open air (Fig. 2d).

Fig. 2 | Dynamic property of thiourea network. a, The exchange reaction of the model compound. b, c, Infrared spectra of the thiourea network (without reprocessing) before and after thermal oxidization in nitrogen(b) and air(c) for 48 hours at 140 °C.
Based on the mechanism above, a deep picture of the reprocessing behavior of the thiourea based elastomers has been illustrated in Fig.3. The reprocessing includes three steps: grinding, reprocessing and conditioning (Fig. 3a). The “conditioning” is the most important step since it helps to transform hindered thiourea into urea and strengthen the materials. As the conditioning time elongates, the increase of both Young’s modulus and strength has been observed. After 18 hours, the modulus reached a maximum value (Fig. 3b). The mechanical change is accompanied by the chemical change (Fig. 3c). Indeed, the conversion of hindered thiourea urea follows a similar trend as mechanical properties do, further confirming the intrinsic mechanism (Fig. 3d). Moreover, the elastomers could go through multi-times reprocessing without any mechanical drop (Fig. 3e, Fig. 3f).
This work has pointed out a potential solution for the plastic waste. From scientific standpoint, the most valuable contributions of this work lay in two parts: Firstly, the negative effects of side reactions entangled with reprocessing could be counteracted by constructive reactions. By taking suitable constructive reactions into the reprocessing, mechanical deterioration could be avoided despite how complex other side reactions are. Secondly, by embedding special chemical structures into polymer networks, reprocessing could alter the chemical structure of the network, offering a chance to enhance the mechanical behavior of the materials. The “special chemical structures” should not restrict to the thiourea chemistry. On the contrary, there is a bunch of chemical candidates that are able to achieve the same behavior. For example, traditional antioxidant could suppress oxidation by reacting with oxygen. Once similar structure was introduced into the network, the functions of these “inserted antioxidants” may be far beyond just alleviating oxidation. The above discoveries have indicated that the mechanical deterioration of reprocessed polymers could be overcome by rational network design at the very beginning of polymer synthesis.
This work has pointed out a potential solution for the plastic waste. From scientific standpoint, the most valuable contributions of this work lay in two parts: Firstly, the negative effects of side reactions entangled with reprocessing could be counteracted by constructive reactions. By taking suitable constructive reactions into the reprocessing, mechanical deterioration could be avoided despite how complex other side reactions are. Secondly, by embedding special chemical structures into polymer networks, reprocessing could alter the chemical structure of the network, offering a chance to enhance the mechanical behavior of the materials. The “special chemical structures” should not restrict to the thiourea chemistry. On the contrary, there is a bunch of chemical candidates that are able to achieve the same behavior. For example, traditional antioxidant could suppress oxidation by reacting with oxygen. Once similar structure was introduced into the network, the functions of these “inserted antioxidants” may be far beyond just alleviating oxidation. The above discoveries have indicated that the mechanical deterioration of reprocessed polymers could be overcome by rational network design at the very beginning of polymer synthesis.
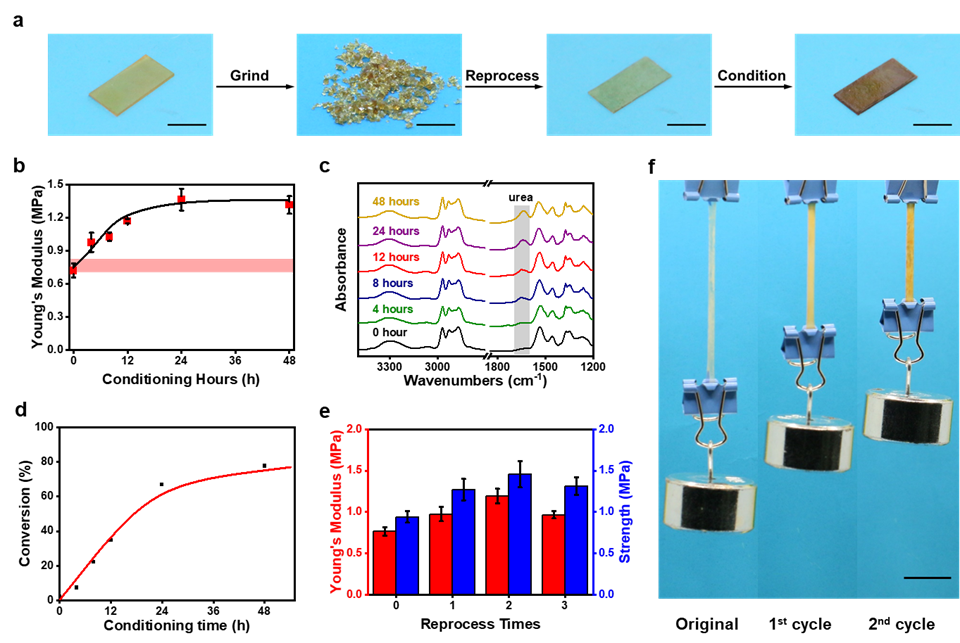
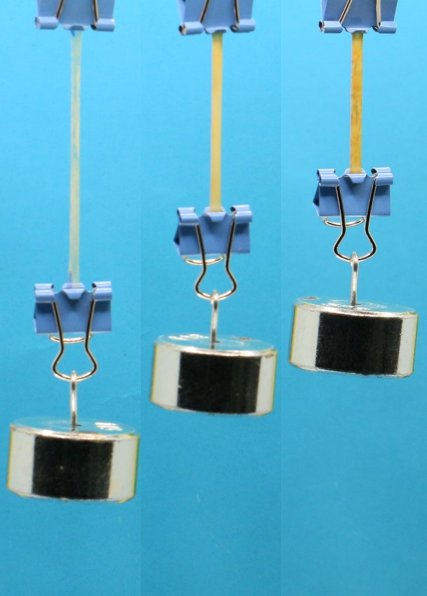
Fig. 3 | Network reprocessing and enhancement in mechanical properties. a, Photographic images of the sample at different stages during the reprocessing. b, Change in Young’s modulus of the reprocessed samples with different conditioning time in air. c, FTIR change of the reprocessed samples with different conditioning time (in air) d, Hindered thiourea conversion of the reprocessed samples with different conditioning time. e, Change in Young’s modulus and strength at different reprocessing cycles. For each reprocessing cycle, the conditioning time is 4 h. f, Photographic images of the thiourea sample with different reprocessing cycles under a constant load of 50 g. The sample size without the external load is 15×3×0.5 mm. All scale bars are 1 cm.
Related work: https://doi.org/10.1038/s41467-022-28085-2
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in