Urgency and Necessity of Epstein-Barr Virus Prophylactic Vaccines
Published in Healthcare & Nursing
Explore the Research
Urgency and necessity of Epstein-Barr virus prophylactic vaccines - npj Vaccines
npj Vaccines - Urgency and necessity of Epstein-Barr virus prophylactic vaccines
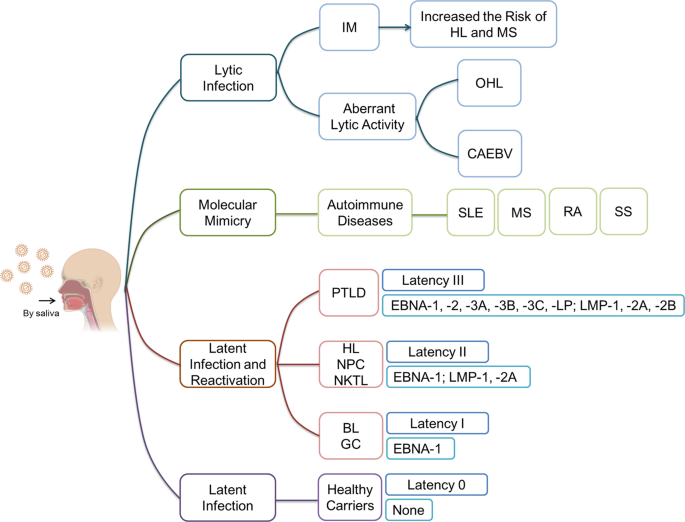
EBV infects more than 95% of humans and establishes a lifelong infection. EBV causes heavy global public health burdens. As reported, EBV caused 75,000 new cases/year of IM in the USA and 113,205, 105,554, 40,109 and 6,318 new cases/year of GC, NPC, HL and BL worldwide, respectively. It is very necessary and urgent to develop an EBV prophylactic vaccine to prevent EBV infection and reduce the burden of all its associated diseases.
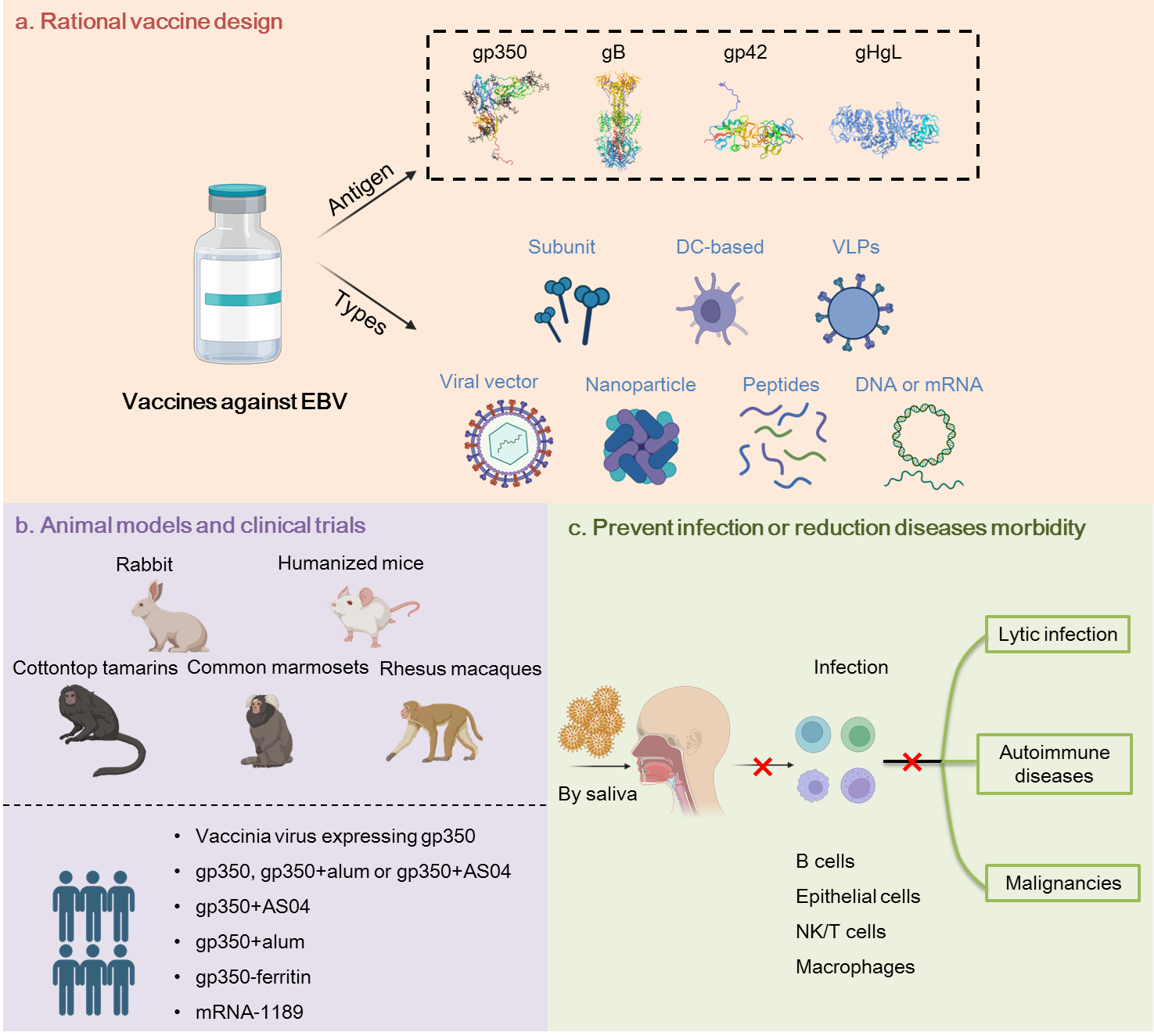
Currently, none of the vaccine candidates is approved for clinical use, despite multiple attempts to develop an effective vaccine. Subunit vaccines, epitope vaccines, DNA vaccines, protein scaffold-based vaccines, viral vector vaccines, VLPs and DC vaccines, all generated important information but generally failed to induce the required level of protection.
Current efforts focus on antigen selection, combination and design to improve the efficacy of vaccines. EBV glycoproteins such as gH/gL, gp42 and gB show excellent immunogenicity in preclinical studies compared to the previously favored gp350 antigen. Combinations of multiple EBV proteins in various vaccine designs become more attractive approaches considering the complex life cycle and complicated infection mechanisms of EBV. Besides, rationally designed vaccines such as virus-like particles (VLPs) and protein-scaffold based vaccines elicited more potent immune responses than soluble antigens. Recently, clinical trials have been luanched to evaluate an mRNA vaccine (mRNA-1189) containing four mRNAs encoding gH, gL, gp42 and gp220 (NCT05164094) and a nanoparticle vaccine ferritin-gp350 (NCT04645147). Besides, suitable animal models also need to be investigated and improved for efficacy evaluation. Humanized mice, rabbits, rhesus macaques and common marmosets are the most common animal models. However, each one of them has obvious limitations.
In the completed human clinical trials, all vaccine candidates failed to prevent EBV infection. Induction of sterile immunity significantly correlates with a reduction of EBV-associated diseases. Hence, the ultimate goal of researchers is still to generate a sterile immunity. However, preventing EBV-associated diseases occurrence rather than EBV infection remains a valuable outcome when completely preventing EBV infection is not achieved. Long-term clinical trials will be needed to assess the ability of EBV vaccine to limit EBV-related diseases, in particular malignancies. Vaccination may also induce more potent cellular immune responses to control EBV reactivation in infected individuals. Thus a vaccine that limits reactivation frequency and severity will show valuable protective effect in infected individuals.
Induction of robust, long-term and balanced humoral and cellular immune responses should remain the primary goal in the development of a protective EBV vaccine. The antigen spectrum, the immunogenicity of selected antigens and the breadth of immune responses are the key issues to achieve this goal. Over the past years, immunogen selection has changed from glycoproteins, especially gp350, to a more extensive range including lytic and latent proteins. The identification and characterization of B and T cell epitopes of EBV protein aid to further optimize immunogen design. Nanoparticle-based systems showed potential for vaccine development and novel adjuvant formulations are promising to increase immunogenicity. In addition, antibody-guided vaccine design also provides a framework to improve EBV vaccine development based on the knowledge of EBV-neutralization acquired over many years.


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in