Using Genetic Algorithms to more Efficiently Optimize the Synthesis of Metal-Organic Frameworks
Published in Chemistry

Al-PMOF (Al2(OH)2TCPP) [H2TCPP = meso-tetra(4-carboxyphenyl)porphine] (Fig. 1) was recurrently studied in my group for different projects. However, when we carried out the standard synthesis in a programmable oven in pure water,1 we would obtain vastly different yields. Moreover, this structure’s relatively long reaction time (i.e., 16 hours) presented a bottleneck for large-scale applications, such as wet-flue gas carbon capture.
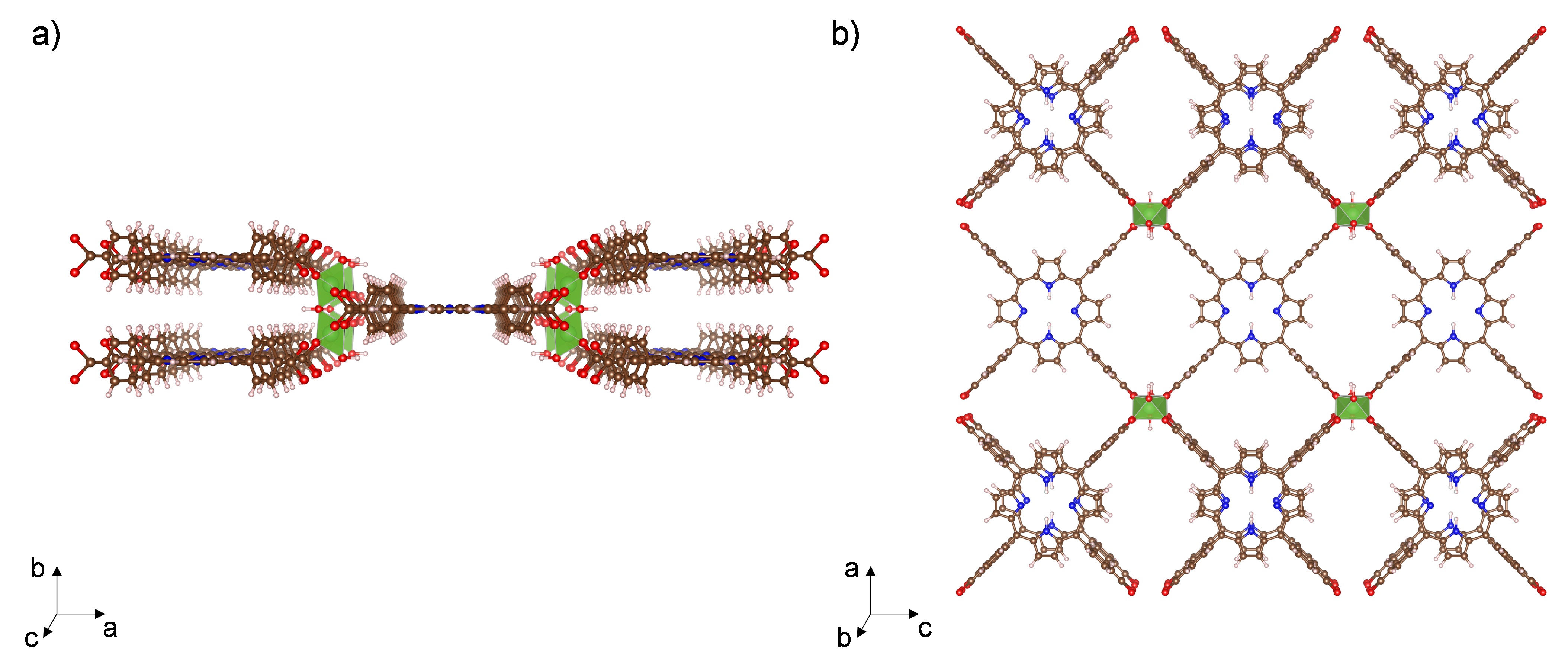
To address these points and improve the synthesis of Al-PMOF, we used a machine-learning approach that employs a genetic algorithm to systematically look for the optimal synthesis conditions (Synthesis Conditions Finder – SyCoFinder).2 We carried out the syntheses in a microwave-based robotic platform, and were thus able to run reactions sequentially, and produce MOFs at a considerably higher rate using an easily-automated process. To optimize the synthesis, we decided to study several parameters that could affect the outcome of the reaction, including:
- Microwave power;
- Reaction temperature;
- Reaction time;
- Concentration of precursors;
- Solvent.
After we defined the importance of each variable and range in which we wanted them to vary, the SyCoFinder web application provided us with the first set of experimental synthesis conditions (1st generation). The samples from the 1st generation were synthesized, collected, and analyzed such that we would be able to answer the following questions: is this the MOF structure we are looking for? How crystalline is it? These were addressed via powder X-ray diffraction (PXRD). Throughout the first set of experiments, we could observe a significant discrepancy in the results. Some reactions did not yield any powder, others were amorphous, while the remaining ones were crystalline and matched the calculated PXRD from the CIF of Al-PMOF (Fig. 2).
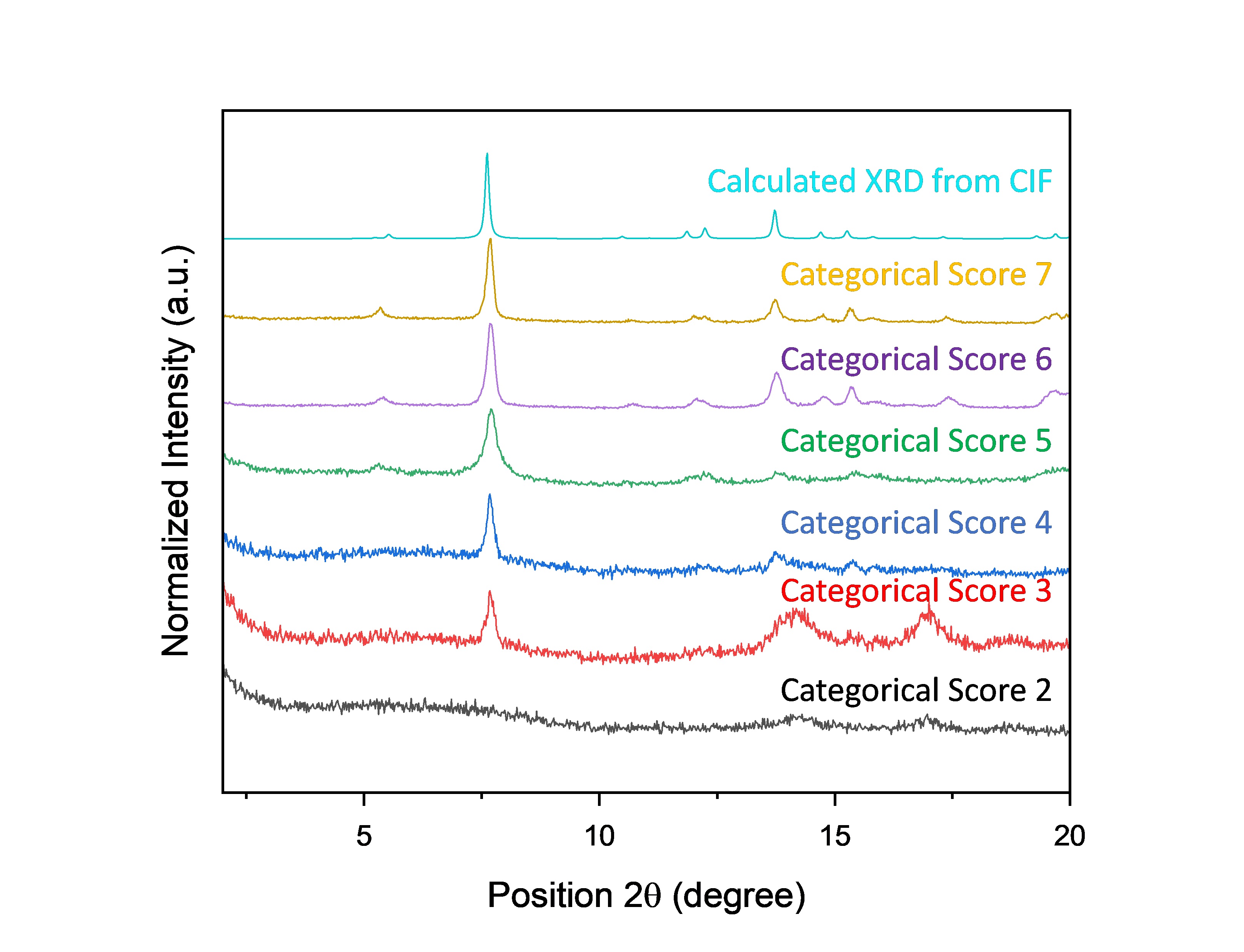
We could therefore rank the samples in terms of their crystallinity from 1 (worst) to 10 (best) and input these rankings back into the web application of the SyCoFinder. The algorithm processes the data, learns from the “failed” and successful experiments, and provides a new set of synthesis conditions (2nd generation) to carry out in the lab. A machine learning approach can more easily build a quantified chemical intuition, similar to the high level of expertise of chemists in the lab, without the need for large datasets.
The 2nd generation of reactions were performed in the lab and ranked as a function of the PXRD. Since all samples were deemed to be crystalline, we also decided to evaluate the yield, which was highly variable depending on the conditions (between zero and almost 80%) (Fig. 3).
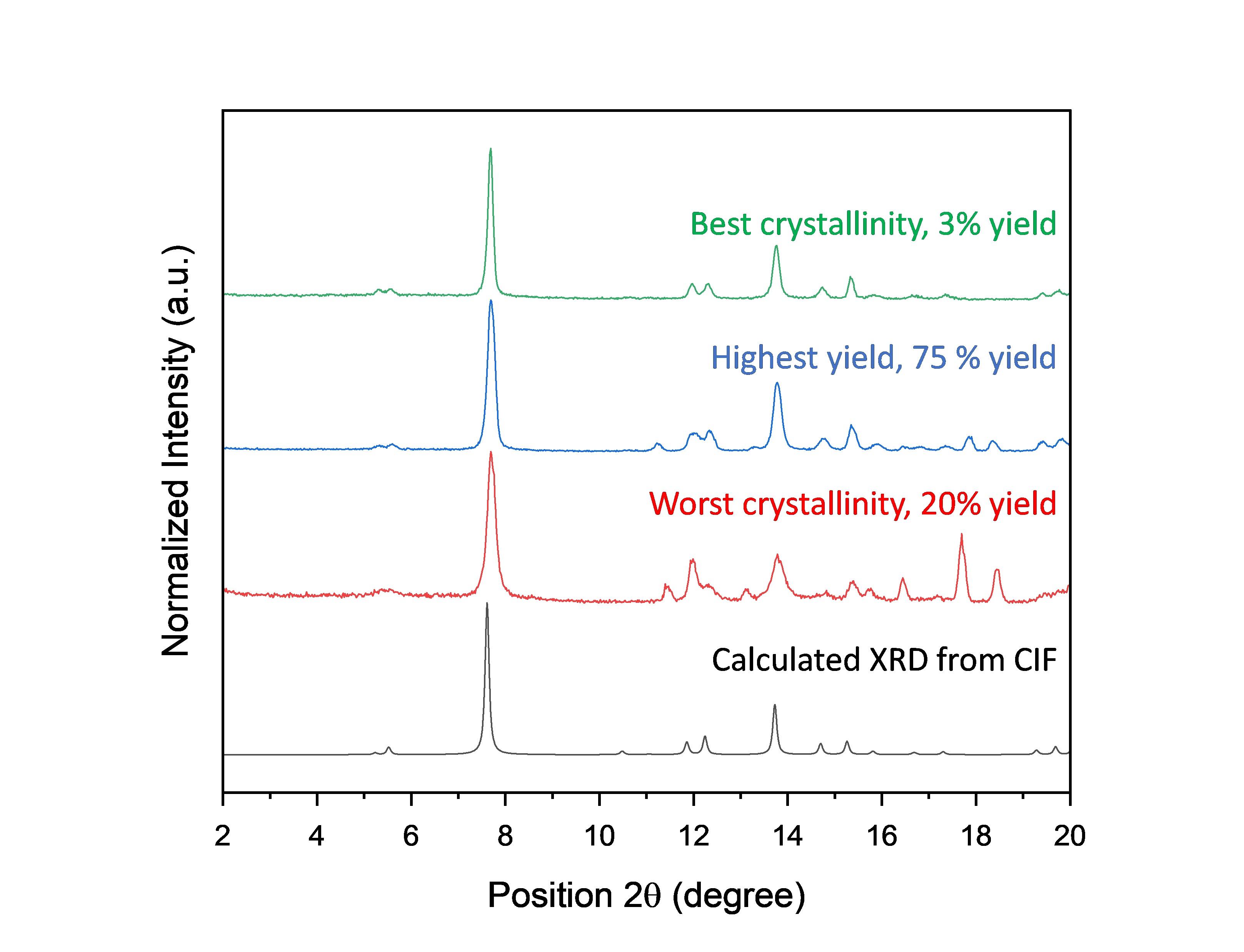
The design of our algorithm makes it easy to expand to different optimization problems, as it constantly factors in our feedback for every new set of conditions. Using the SyCoFinder and the robotic platform this way can help synthesize grams of material substantially faster than with the traditional MOF synthesis equipment.
Crucially, this work highlights the importance of saving all data. We, chemists, learn as much from our successful experiments as from our “failed” experiments. Machine learning is not that different, but if we continue to publish only our successful data, we miss an enormous opportunity to learn from the collective knowledge embedded in all our “failed” experiments.3
1. Fateeva, A. et al. A water‐stable porphyrin‐based metal–organic framework active for visible‐light photocatalysis. Angew. Chem. Int. Ed. 51, 7440-7444 (2012).
2. Moosavi, S., Talirz, L. & Smit, B. Synthesis conditions finder. https://www.materialscloud.org/work/tools/sycofinder. Zenodo https://doi.org/10.5281/zenodo.2554380 (2019).
3. Jablonka, K. M., Patiny, L. & Smit, B. Making the collective knowledge of chemistry open and machine actionable. Nat. Chem. 14, 365-376 (2022)
Follow the Topic
-
Communications Chemistry

An open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of the chemical sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Experimental and computational methodology in structural biology
Publishing Model: Open Access
Deadline: Apr 30, 2026
Advances in Asymmetric Catalysis for Organic Chemistry
Publishing Model: Open Access
Deadline: Mar 31, 2026

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in