Using Nanoparticle “Backpacks” to Rejuvenate Damaged Stem Cells
Published in Bioengineering & Biotechnology
In 2016, when I was a postdoctoral fellow at Harvard Medical School, my personal and professional lives intersected in a surprising way. I was researching the ways that stem cells help rebuild tissues and blood vessels. At the same time, my wife became pregnant with our first child. Naturally, I was excited by the new trend of parents choosing to store, or “bank,” their infant’s stem cell-rich umbilical cord blood in case it might prove useful for treating an unexpected illness later in life.

But plans changed suddenly when my wife became very sick. At 37 weeks pregnant, she was told she would need to deliver our baby right away due to preeclampsia.
Fortunately for us, the pregnancy was considered in early full term, and my wife and our daughter were fine. But we were told that our daughter’s umbilical cord blood would not be worth saving. As a father and as a stem cell researcher, I was crestfallen. However, the experience also suggested new research questions: What exactly happens to stem cells affected by complicated pregnancies—and what, if anything, might be done to rejuvenate the stem cells once they have been damaged?
Six years later, our study forthcoming in Communications Biology, has provided some initial answers.

A Closer Look at Damaged Stem Cells
We collaborated with the “Hoosier Moms” Study at the Indiana University School of Medicine to collect hundreds of samples of damaged stem cells. These particular stem cells had been damaged not by preeclampsia but by another, even more common pregnancy complication: gestational diabetes mellitus (GDM), which occurs in 6-15% of all pregnancies.
While clinicians typically find that mothers make a full recovery from GDM, it is less clear how the complication affects the infant. We were able to observe in great detail the ways these stem cells differ from healthy stem cells, both in movement and function. This allowed us to provide an updated model for the way a complicated pregnancy might continue to affect the health of the infant over the course of his or her life. Importantly, we were also able to locate a specific molecule to target in order to correct the damaged stem cells.

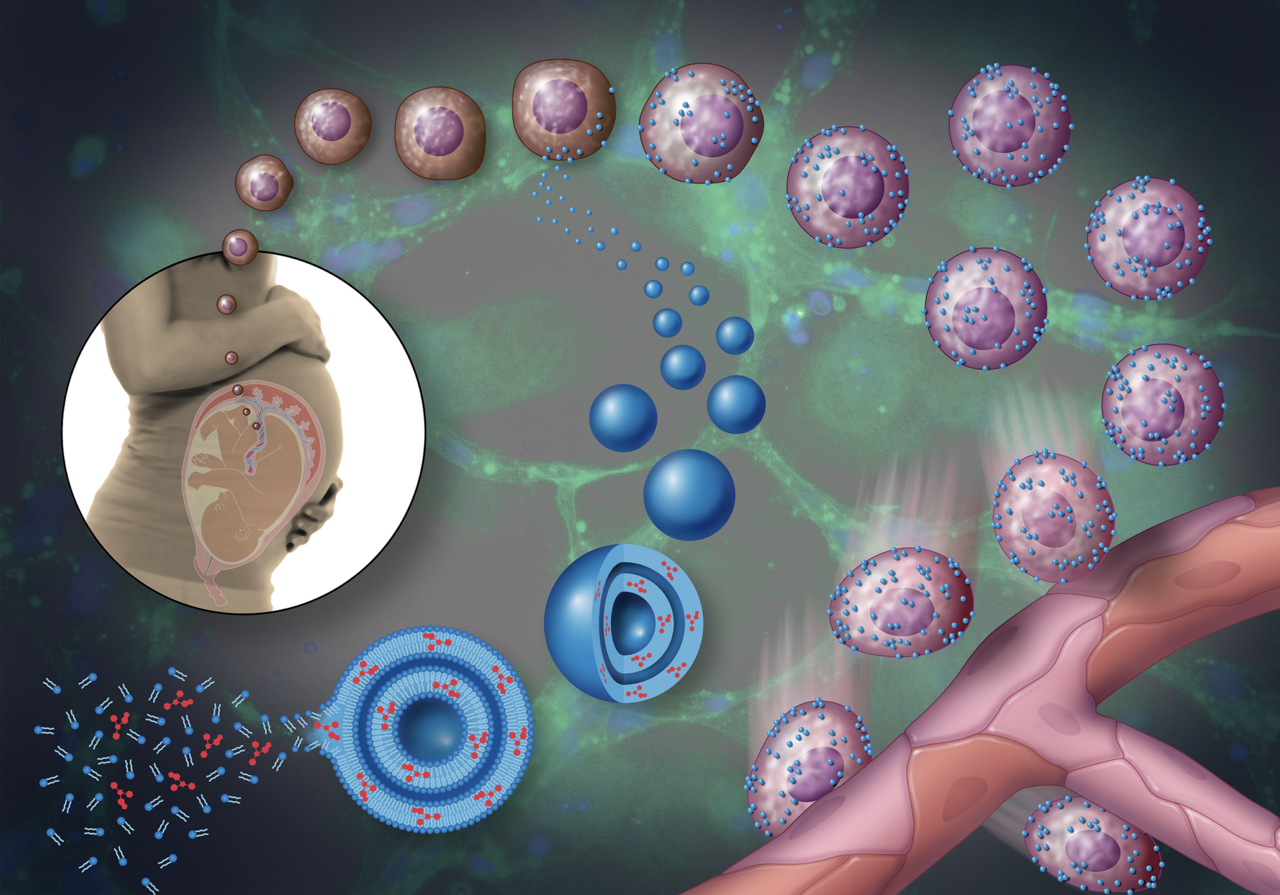
Building “Backpacks”
Now came the hard part. Prior to any treatment, the stem cells damaged by GDM moved slowly and were unable to form tissues. Could we deliver medication directly to the cells in order to make them function again?
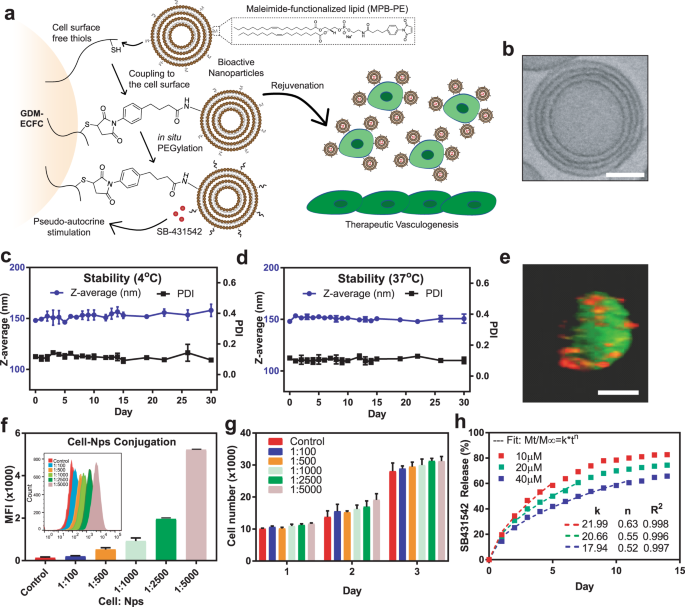
We were inspired by previous studies that used nanoparticles attached directly to cells as a drug delivery system. The analogy that has become common in the field is to think of the cell as a “soldier.” The soldier is smart and highly capable, but the soldier—especially one who is injured or weakened—can benefit from a “backpack” that makes the soldier more effective. A group of researchers at Harvard had found, for example, that they could equip immune cells with cytokine-loaded backpacks that made them more effective in destroying cancerous tumors. Our idea was to modify this approach for use with stem cells.
To build the “backpacks,” we create liposomes, permeable spheres that each measure about 150 nanometers in diameter. We formulated, or “tuned” the liposomes so they would release medication slowly, over about a two-week period. We infused the liposomes with the medication along with a dye that allowed us to observe them on the surface of the stem cells. We mixed the finished “backpacks” together with stem cells and flushed away any that did not stick to the surface of the cells.
We then observed the “backpack”-equipped stem cells in two environments: both in vitro in a collagen gel and also in vivo, under the skin of lab mice. We were not surprised at all to find that the stem cells began to form blood vessels in the collagen gel. But we were less certain about what would happen in vivo. We were very excited, though, to find that the treatment worked in the samples in the mice as well.

From the Lab to the Clinic
We are optimistic that our technology and strategy for regenerating stem cells has a clear path to clinical settings, especially compared with other approaches. Methods that involve injecting the medicine directly into the bloodstream come with many unwanted risks and side effects, and new methods like gene-editing face a long road to Food and Drug Administration (FDA) approval. Our strategy used only methods and materials already approved for clinical settings by the FDA.
Often, the infants born from complicated pregnancies are those who could most stand to benefit from stem cells, but, unfortunately, these are the same infants whose stem cells we currently discard. For example, a baby born prematurely due to preeclampsia may have to stay in the NICU with an imperfectly formed lung. We hope our technology can improve this child’s developmental outcomes. Instead of discarding the stem cells, in the future we hope clinicians will be able to rejuvenate them and use them to regenerate the body. And in our current work, my lab is looking in more detail at ways to target particular organs for regeneration.
Acknowledgments
We would like to thank Brett Beasley, writer and editorial manager at Notre Dame Research for writing the story, and Wes Evard from Notre Dame Engineering Graphics for the pictures.
The study was made possible by funding from Notre Dame’s Advancing Our Vision Initiative in Stem Cell Research, Notre Dame’s Science of Wellness Initiative, the Indiana Clinical and Translational Science Institute, the American Heart Association, and the National Institutes of Health.
Find out more about how Notre Dame conducts (non-embryonic) stem cell research in accordance with Catholic ethics at https://stemcell.nd.edu/ethics/.
About Notre Dame Research:
The University of Notre Dame is a private research and teaching university inspired by its Catholic mission. Located in South Bend, Indiana, its researchers are advancing human understanding through research, scholarship, education, and creative endeavor in order to be a repository for knowledge and a powerful means for doing good in the world. For more information, please see research.nd.edu or @UNDResearch.
Follow the Topic
-
Communications Biology

An open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of the biological sciences, representing significant advances and bringing new biological insight to a specialized area of research.
Related Collections
With Collections, you can get published faster and increase your visibility.
Signalling Pathways of Innate Immunity
Publishing Model: Hybrid
Deadline: Feb 28, 2026
Forces in Cell Biology
Publishing Model: Open Access
Deadline: Apr 30, 2026
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in