Viral Protein Coat Linked to Infectivity & Alzheimer's Disease
Published in Microbiology

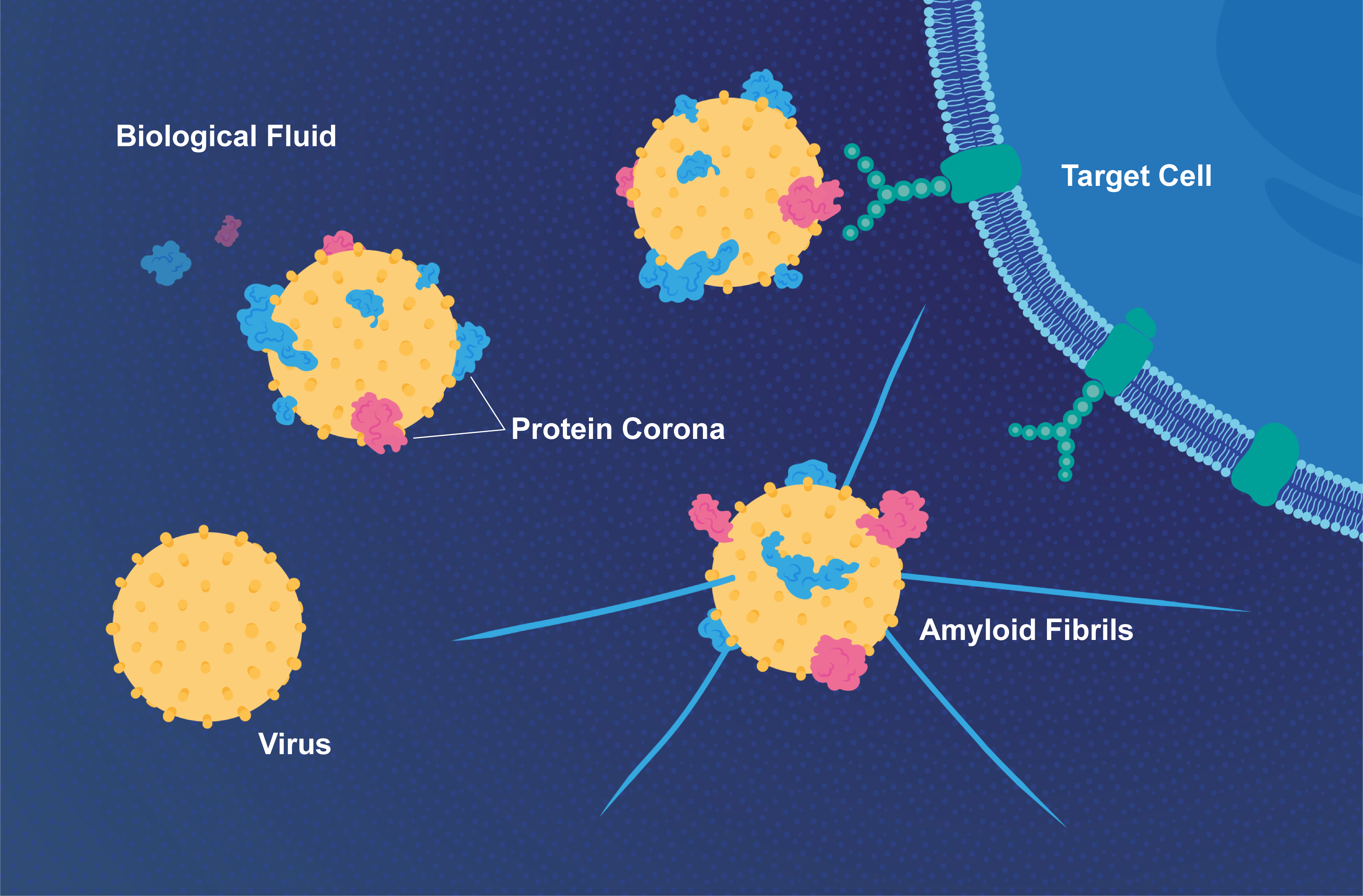
Are viruses dead or alive? Well.. both. Viruses can engage in life-like activities such as replication only inside a living cell, where they exploit the cellular machinery of their host to their benefit. However, before entering a host cell, viruses are just nanometer-sized particles, very similar to artificial nanoparticles that are used in medical applications for diagnosis and therapy. Based on this similarity, we found that viruses and nanoparticles share another important property; they both get covered by a layer of proteins when they encounter the biological fluids of their host before they find their target cell. This layer is called the protein corona, and it coats the surface of nanostructures and significantly influence their biological activity. It is like a a tennis ball falling into a bowl of milk and cereals (Video 1). The ball gets immediately covered by the sticky particles in the mix. The same thing happens when a virus gets in contact with blood or lung fluids for example. These fluids contain thousands of proteins that are soluble or just floating. Many of these proteins immediately stick to the viral surface forming a protein corona.
Video 1.
https://www.youtube.com/watch?v=w27cMbdycDA
To demonstrate both the complexity and importance of the viral protein corona, we studied the protein corona of respiratory syncytial virus (RSV) in different biological fluids. RSV is the most common cause of acute lower respiratory tract infections in young children worldwide, leading up to 34 million cases and 196,000 fatalities each year. We showed the protein corona signature of RSV in the blood is very different from that in lung fluids, and that it is also different between humans and other species such as rhesus macaque monkeys, which get infected with RSV. The virus remains unchanged on the genetic level; however, it acquires different identities by accumulating different protein coronae on its surface depending on its environment. This gives the virus versatility to utilize extracellular host factors for its benefit, and indeed, we show that many of these different coronae enhance RSV infectivity.
One interaction with a special class of extracellular host proteins was particularly interesting, namely corona interactions involving proteins that have the tendency to aggregate in pathological conditions such as Alzheimer’s disease (AD). These proteins, termed amyloid proteins, clump forming amyloid plaques that ultimately lead to neuronal cell death. Viruses have been implicated in amyloid pathologies such as AD before; however, the lack of a mechanistic correlation between the two seemingly distinct types of pathology has rendered such connection elusive. In this work, we found that viruses such as RSV and herpes simplex virus type 1 (HSV-1) can bind amyloid proteins on their surface and dramatically accelerate their aggregation into the fibrillar structures characteristic of amyloid plaques. The mechanism is called surface-assisted heterogeneous nucleation, in which viruses act as catalytic surfaces that induce the conversion of soluble proteins into insoluble ones. This conversion or phase transition is not unique for amyloid proteins, it happens in many other natural phenomena that involve similar transitions. One example is crystallization, where the process of transition from a supersaturated solution into organized crystals is dramatically accelerated by the introduction of exogenous surfaces (metal beads) or preformed crystals (Video 2).
Video 2.
https://www.youtube.com/watch?v=NAPrI_ArkRk
We found that HSV-1 is able to catalyze the process of amyloid aggregation on its surface leading to amyloid plaque formation in animal models of AD within 48 hours of infection in the brain; a process that typically takes several months to take place in these animal models in absence of HSV-1 infection.
Taken together, or data highlight how viruses manipulate and modulate the extracellular environment of the host to their benefit. With very few genes, viruses can still enrich their surface properties by selectively binding protein corona factors from the biological fluids of the host to enhance their infectivity. Additionally, viruses are able to catalyze the phase transition of certain proteins from soluble into insoluble forms leading to amyloid protein aggregation that is implicated in many neurodegenerative disorders. The novel mechanisms described by this paper can have an impact not only on understanding determinants of viral tissue selectivity (tropism) and transmission, but also on devising new ways to produce viruses in cell culture and designing new vaccines that take into consideration the protein corona factors. In addition, describing a physical mechanism that links viral and amyloid pathologies adds weight to the increasing research interest in the role of microbes in neurodegenerative disorders such as AD and opens up new avenues for therapeutic interventions.
The article
“The Viral Protein Corona Directs Viral Pathogenesis and Amyloid Aggregation” by Ezzat et al is published in Nature Communications [https://www.nature.com/articles/s41467-019-10192-2]
More information
Kariem Ezzat, Department of Molecular Biosciences, The Wenner-Gren Institute, Stockholm University and Department of Laboratory Medicine, Karolinska Institutet. Phone: + 46 8 16 14 37, mobile: +46 720 39 09 93, e-mail: kariem.ezzat@su.se
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in