Vitamin B12 uptake by gut bacteria: surprises beyond E. coli.
Published in Microbiology

Background:
Despite of decades studying nutrient acquisition in bacteria, our knowledge is mostly derived from Proteobacteria, leaving this key process poorly characterised in other important bacterial groups. Briefly, uptake is defined as the translocation of nutrients from the external space to the cytoplasm and for this, nutrients need to cross the cell envelope. For Gram-negative bacteria this “crossing” becomes more challenging due to the presence of an extra layer in that envelope, the outer membrane (OM; Figure 1). This external feature is a terrific asset due to its intrinsic barrier function which protects the cell from noxious compounds. However, the price to pay for this protection is a more complex nutrient acquisition process. To solve this problem, Gram-negative bacteria have two classes of OM transport proteins: diffusion channels (eg. porins) and active transporters (TonB-dependent transporters, TBDTs; Figure 1). The former transport small molecules by an inwardly-directed concentration gradient, while TBDTs are active transporters that import larger molecules and utilise very low external concentrations of substrate. These scarce molecules, for example B12 or iron, play key roles in a multitude of cellular processes such as colonisation of the environment, survival and pathogenesis, and are therefore valuable to the cell. One of the best studied TBDTs is BtuB from Escherichia coli, which imports the most complex vitamin in nature, B12 (cobalamin).
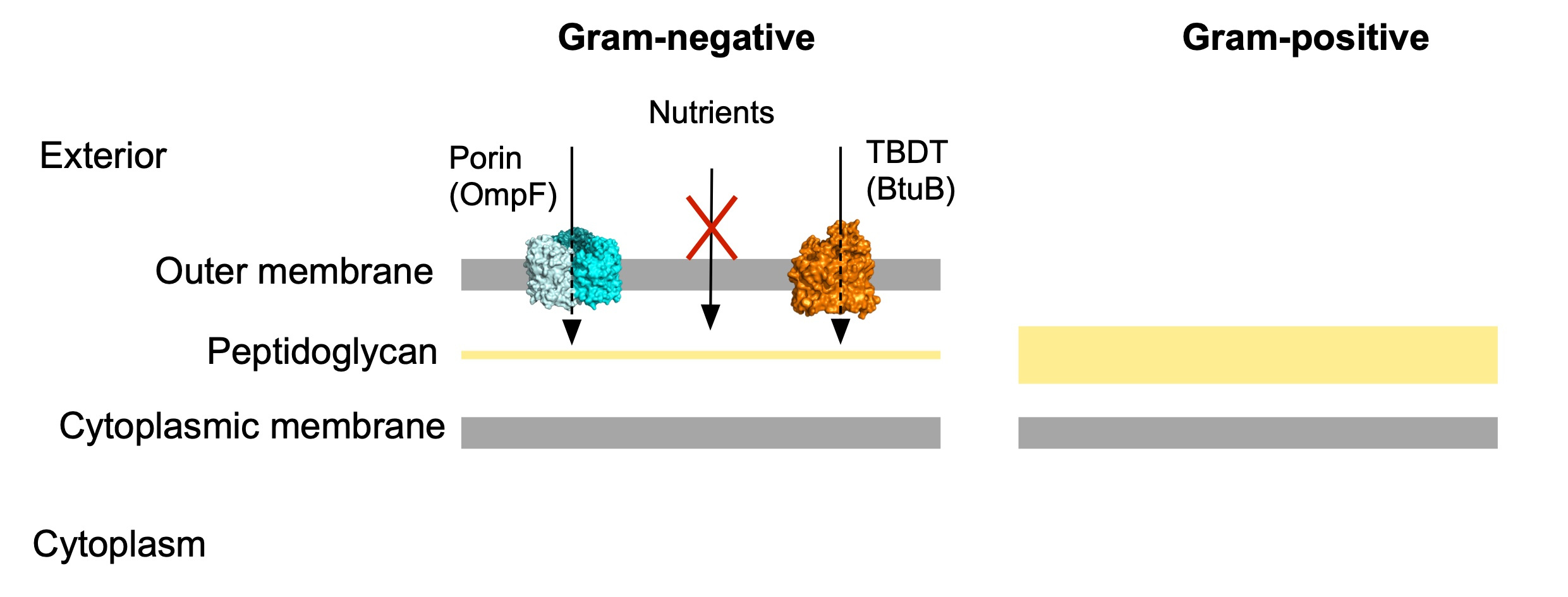
Figure 1. Schematic representation of the cell envelopes of Gram-negative and Gram-positive bacteria. Cytoplasmic membrane transporters are not shown.
Why study B12 uptake?
B12 is essential for many organisms, including bacteria and humans, but it is only produced by a selected group of microorganisms. Being such an important vitamin in high demand and low availability, I wondered if the transport mechanism characterised in E. coli differs in other bacterial groups. This question was based on two premises: - I was working at the Bert van den Berg lab in which one of the lines of research focuses on understanding uptake of complex glycans in the prominent gut bacteria Bacteroides thetaiotaomicron (B. theta, a distant organism to Proteobacteria). Bert’s lab showed that TBDTs implicated in glycan acquisition form stable complexes (termed utilisomes) with OM lipoproteins. - At the same time, Andy Goodman’s lab (Yale University) described that B. theta has several OM lipoproteins (BtuG and BtuH) which bind B12 and offer a selective advantage for this bacterium. So this led me to wonder, how are these lipoproteins working with BtuB to take up B12? Are they forming a complex similar to the utilisome described for glycans?
Our results:
B. theta has three different BtuBs, adding an extra level of complexity to this study. However, the most challenging issue we faced was to obtain enough protein for the structural studies, because B. theta OMPs generally do not express recombinantly in E. coli. Expression of BtuB is negatively regulated by B12, so we had to replace the B12-dependent promoters with a constitutive promoter. The purification of each BtuB (directly from B. theta) showed that they form stable complexes with their respective B12-binding protein partner BtuG. Our structures only showed closed complexes in which BtuG is capping BtuB in a fashion that resembles a pedal-bin (Figure 2a), but it is clear that to accept B12 these complexes have to open.
Although obtaining the structures for the complexes was very exciting, for me the most puzzling question was still unresolved. BtuG binds B12 with sub-picomolar affinity, prompting the question "how is B12 transferred from BtuG to BtuB?". To tackle this, we managed (after substantial effort) to obtain a BtuBG complex loaded with B12, and solved the structure via cryo-EM. Surprisingly the structure of BtuG-B12 is virtually the same in complex with BtuB or alone (ruling out a conformational change in BtuG, upon closing of the complex, potentially promoting vitamin release) but the cryo-EM data showed different subgroups of particles in which an external loop (EL8) from BtuB was ordered or disordered. In this cryo-EM structure this loop is in the vicinity of the BtuG B12 binding pocket while in the apo-BtuBG crystal structure this loop occupies the B12 binding pocket. This suggested that once B12 is bound by BtuG, the complex closes and BtuB EL8 pushes B12 out of its BtuG binding pocket, perhaps similar to a spring-loaded hinge. Gratifyingly, this hypothesis was confirmed with functional and molecular dynamics studies, solving the conundrum of how B12 is displaced from a very high-affinity binding site in BtuG to move towards BtuB, followed by uptake (Fig. 2). Overall, our study showed that lipoproteins assist the TBDT-mediated uptake of both B12 and glycans, suggesting that (i) this confers a competitive advantage, and (ii) most if not all active uptake processes are lipoprotein-assisted in Bacteroides species.
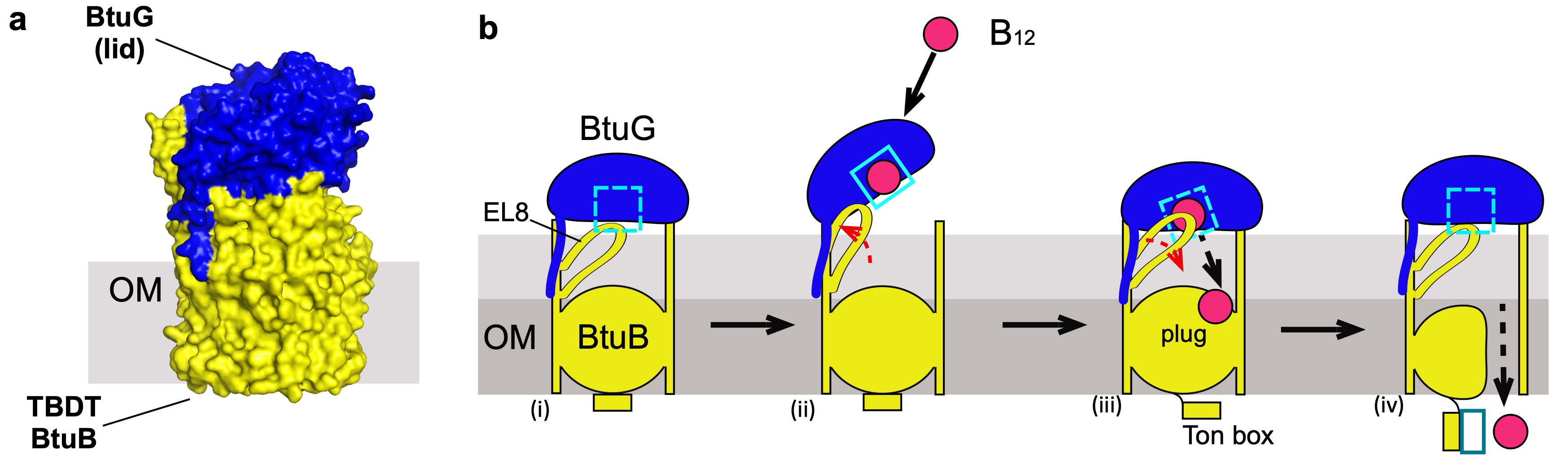
Figure 2. a, surface representation of the crystal structure of the complex BtuB (yellow)-BtuG (blue). b, proposed model of B12 uptake, (i) closed complex, EL8 occludes the binding site; (ii) the complex opens, EL8 is displaced and B12 binds BtuG; (iii) upon closing, EL8 returns to its natural position forcing the displacement of B12; (iv) B12 is translocated into the periplasm.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in