Vitamin C: abrupt termination may increase mortality of sepsis patients
Published in Healthcare & Nursing
In animal models of sepsis, vitamin C prevented hypotension and lung injury, improved capillary blood flow, and prolonged survival. Two randomized trials reported that vitamin C significantly decreased mortality for patients with sepsis (Zabet 2016; CITRIS-ALI 2019). In the CITRIS-ALI trial, there was significant time-dependent modification of the vitamin C effect, with decreased mortality during the 4-days of vitamin administration, but not after vitamin C was stopped (Hemilä 2020). We also used the quantile treatment effect approach to estimate the effect of vitamin C in the CITRIS-ALI trial and found that, for example, the proportion of patients alive after 5 days in the placebo group was the same as that after 13 days in the vitamin C group, also demonstrating the benefit of vitamin C (Hemilä 2022).
The recent LOVIT 2022 trial, including 862 participants, further examined the potential effects of vitamin C on sepsis patients. Unexpectedly, those in the vitamin C group had 1.17 times the risk of dying within 28 days than those in the placebo group. The LOVIT authors concluded that “those who received intravenous vitamin C had a higher risk of death or persistent organ dysfunction at 28 days than those who received placebo”.
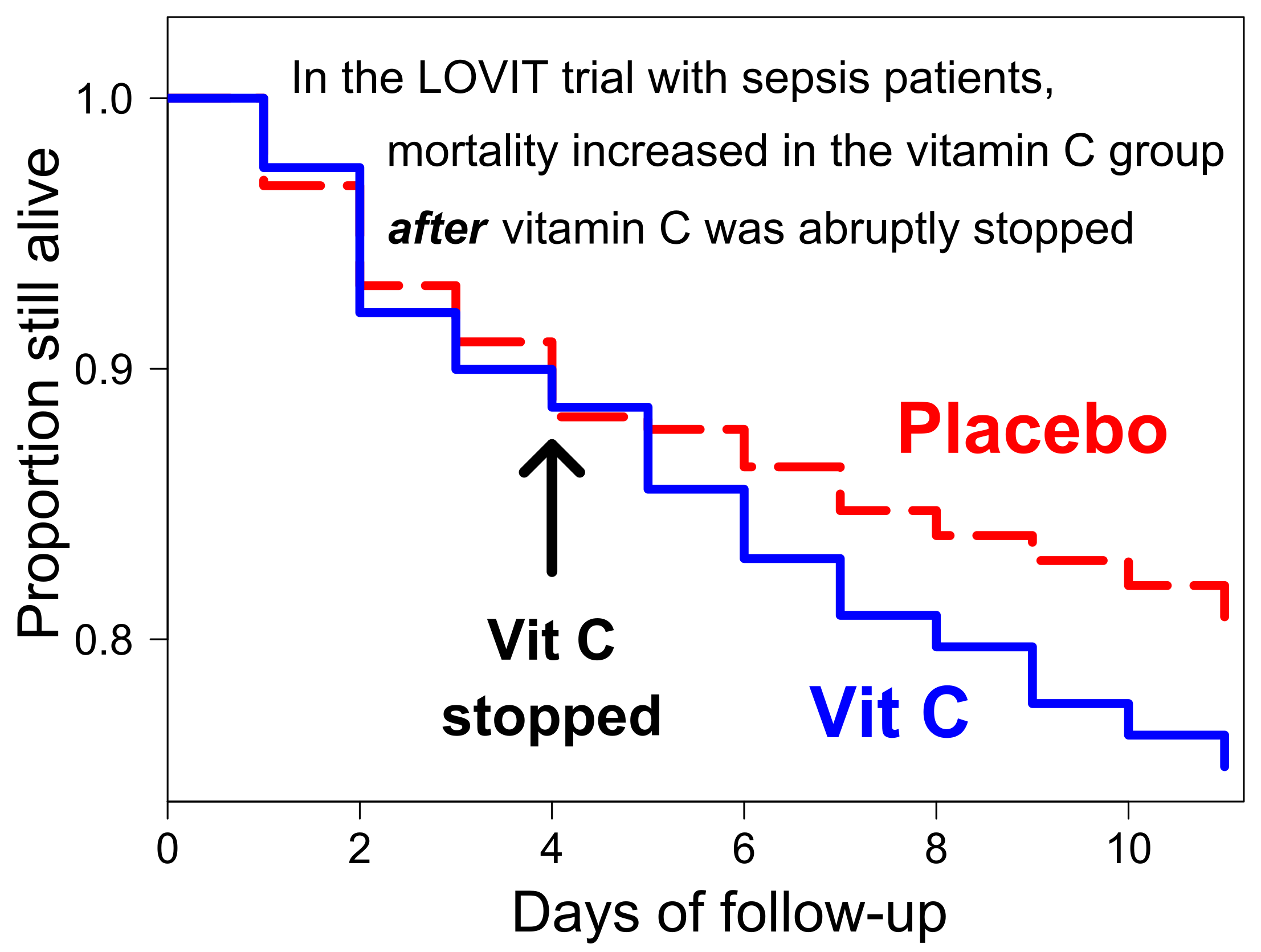
However, in the LOVIT trial vitamin C administration was just 4 days, whereas the median length of ICU stay was 6 days, that is, 2 days longer than the vitamin C administration. In addition, 25% of patients stayed in ICU for more than 12 days, that is more than 8 days after vitamin C was stopped. Given that the effects of vitamin C administration are likely to be short-lasting, we investigated whether the survival difference between the vitamin C and placebo groups was modified by the duration of vitamin C administration.
We found that there was no difference between the vitamin C and placebo groups during the 4-day vitamin C administration, indicating that vitamin C itself was not harmful. Immediately after the termination of vitamin C, on days 5 to 7, there was a 2.3-fold increase in mortality in the vitamin C group when compared to the placebo group, see Figure below. This indicates that it was the termination of vitamin C which caused the harm to the vitamin C group. Hence the conclusion of the LOVIT trial authors that the “receiving” of vitamin C explained the observed harm seems unjustified. Further research on the role of vitamin C for critically ill patients is needed and should not be discouraged by the increased mortality caused by the abrupt termination of vitamin C in the LOVIT trial.
There has been significant bias against vitamin C for many years. However there is a substantial body of evidence that vitamin C can be beneficial in certain circumstances, such as leading to shorter ICU stay and ventilation time, increasing low LVEF levels, and decreasing the risk of AF. Given its low-cost, ready availability and safety, it seems obvious that further research is warranted.
Follow the Topic
-
European Journal of Clinical Nutrition

An international, peer-reviewed journal covering all aspects of human and clinical nutrition. This may encompass clinical, metabolic and epidemiological studies that describe nutritional interventions for clinical disease and health promotion.

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in