What do we really know about IDO1 moonlighting and why is it important?
Published in Chemistry, Physics, and Biomedical Research
What is Protein Moonlighting?
The term moonlighting was introduced by Constance Jeffery in 1999 to define the increasing number of proteins that were reported as having two or more physiologically relevant functions.1 Moonlighting is the result of the versatility of the evolution that makes use of some of the already available proteins to generate new functions, thereby saving energetic resources.
Many metabolic enzymes belong to the class of moonlight proteins. These proteins have evolved non-canonical secondary functions in addition to their canonical (i.e. catalytic) functions, which are confined through the regulation of their localization, expression, or binding partners in a cell. Moonlighting proteins may switch between canonical and non-canonical functions through adopting different conformational states, with each state performing one function, with or without affecting the other function. These conformational states are induced and/or selected through distinct molecular mechanisms including post-translational modifications (PTMs) and/or interactions with binding partners such as small molecules and proteins.2
The Moonlight Property of IDO1.
Indoleamine 2,3-dioxygenase (IDO1) is a metabolic enzyme that catalyzes the metabolic degradation of dietary tryptophan (l-Trp), producing bioactive metabolites.3 Through this cellular function, it plays a regulatory role in a wide range of biological processes, including neuronal functions, immune response in host-pathogen/microbiota interactions and cancer. The enzyme was discovered at the mid of the last century (1957) by Osamu Hayaishi et al.,4 and involved in the maintenance of maternal T cell tolerance at the end of the century by the studies of David H. Munn et al.5 Since then, an increasing body of evidence has thrust IDO1 into the limelight as a regulator of the ‘brakes’ of the immune system in cancer immunosurveillance, with tumor cells exploiting the enzyme's functions to escape from immune attack. Hence, by representing a pivotal immune check-point protein, IDO1 has attracted intense endeavors for the design and synthesis of potent inhibitors as major goal in the development of novel anticancer drugs. As a result of these efforts, some chemical inhibitors have advanced in clinical settings, including navoximod, epacadostat and linrodostat, yet with questioning efficacy.6
In 2011, the pioneering work of Ursula Grohmann and her team at the University of Perugia, led to the discovery that IDO1 is a metabolic enzyme belonging to the class of moonlight proteins.7 Specifically, it was found that, once phosphorylated by other enzymes, IDO1 can trigger a signaling pathway that completely reprograms an immunostimulatory dendritic cell (DC) into a tolerogenic cell. Grohmann’s research team also discovered that, whereas the tolerogenic effects of IDO1’s metabolic functions are transient, a DC reprogrammed via IDO1’s moonlight functions is capable to maintain tolerance and thereby keep in check auto- and anti-tumor immunity over the long term.
Regretfully, the moonlighting functions of IDO1 were not considered during the R&D studies of enzyme inhibitors in the preclinical stages, with the regulation of catalytic activity being the only readout of those studies that left the unaddressed question about whether the IDO1’s moonlight property may or may not affect the inhibitor’s therapeutic efficacy.
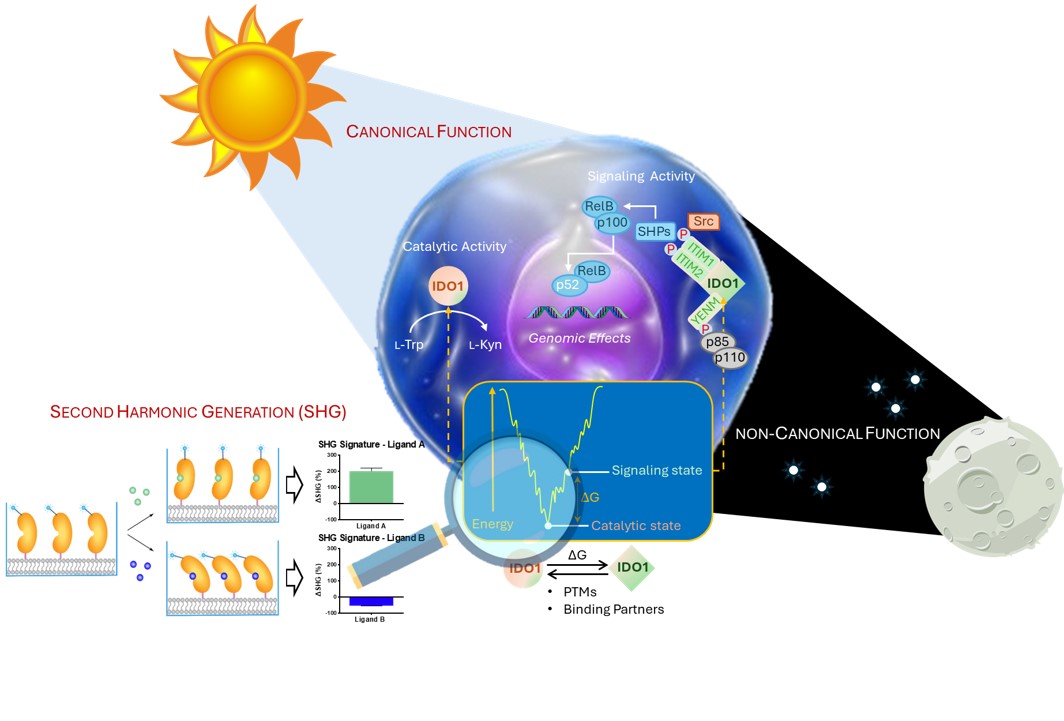
Is It Important for the R&D of IDO1 Inhibitors?
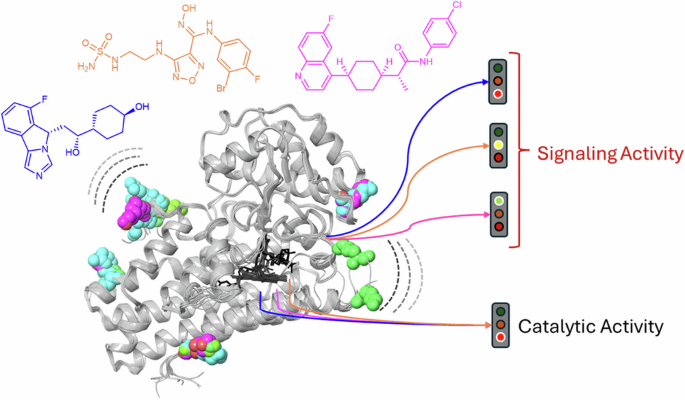
In our recent article by title “Ligand-induced conformations and dynamic allosteric motions of IDO1 affecting the recruitment of a protein signaling partner” we have attempted to address this question, integrating different cutting-edge methodologies to investigate ligand-induced conformations and dynamic allosteric motions of IDO1 for navoximod, epacadostat and linrodostat, namely three different enzyme inhibitors that have reached the clinical setting.
The study started with the development of a biophysical assay for IDO1 based on the second harmonic generation (SHG) biosensor technology. SHG is a nonlinear optical phenomenon wherein two photons of equal energy are combined by a nonlinear molecule or material to emit a single photon with twice the energy. Upon binding to a target protein tethered to a lipid surface, ligands may bind to induce different conformational states of the protein, resulting in different SHG signals. We thought that SHG could provide insightful information on how ligands might regulate the switch between canonical and non-canonical functions of moonlighting proteins by inducing different conformational states (Figure 1).
The SHG biosensor technology was thus applied to detect ligand-induced conformational changes of IDO1 in response to navoximod, epacadostat and linrodostat. Notably, we observed that, by binding to the catalytic site, navoximod produces a decrease of SHG signal, while epacadostat and linrodostat increase the SHG signal of the enzyme, thereby suggesting different ligand-induced conformational states in IDO1.
Molecular dynamics simulations supported the SHG biosensor results, pinpointing differences between the inhibitors in the dynamic allosteric regulation of two regions of the protein located outside the catalytic cleft. Microscale thermophoresis was then instrumental to prove the functional relevance of this regulation by investigating its effect on the stability of the transient signaling complex between IDO1 and Src tyrosine kinase. It was thus found that epacadostat and linrodostat were able to weaken the interaction between IDO1 and Src at odds with navoximod, which strengthened the interaction of IDO1/Src for a more stable transient complex. Overall, these results suggest that the different ligand-induced conformational changes of IDO1 may affect its moonlight functions by regulating the interactions with binding partners such as Src.
Since the signaling activity of IDO1 culminates in a cell-specific transcription of genes, we also demonstrated that distinct transcriptomes are modulated in SKOV3 cells by the three catalytic inhibitors, despite they share a similar ability to inhibit the enzyme’s catalytic function. Accordingly, the inhibitor-induced conformational changes of IDO1 protein, captured by the SHG biosensor technology, do also affect the signal transducing ability of IDO1 in SKOV3 cells, leading to different patterns of gene expression.
Outlook.
Our study provides a template for investigating moonlight proteins by integrating SHG biosensor technology and molecular dynamics. With this regard, it may be desirable to determine as early as possible in drug discovery projects targeting moonlight proteins whether and to what extent ligands change protein’s conformations, affecting their canonical and/or non-canonical functions. Depending on the desired mechanism of action, it will then be possible to selectively tune the inhibitory and signaling properties of a drug candidate using medicinal chemistry via enzyme protein conformational changes as measured by SHG.
References
- Jeffery CJ. Trends Biochem. Sci. 1999, 24(1), 8-11.
- Jeffery CJ. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018, 373(1738), 20160523.
- Pallotta MT, Rossini S, Suvieri C, Coletti A, Orabona C, Macchiarulo A, Volpi C, Grohmann U. FEBS J. 2022, 289(20), 6099-6118.
- Hayaishi O, Rothberg S, Mehler AH, Saito Y. J. Biol. Chem. 1957, 229(2), 889-96.
- Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, Brown C, Mellor AL. Science. 1998, 281(5380), 1191-3.
- Le Naour J, Galluzzi L, Zitvogel L, Kroemer G, Vacchelli E. Oncoimmunology. 2020, 9(1), 1777625.
- Pallotta MT, Orabona C, Volpi C, Vacca C, Belladonna ML, Bianchi R, Servillo G, Brunacci C, Calvitti M, Bicciato S, Mazza EM, Boon L, Grassi F, Fioretti MC, Fallarino F, Puccetti P, Grohmann U. Nat. Immunol. 2011, 12(9), 870-8.
Follow the Topic
-
Communications Chemistry

An open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of the chemical sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
f-block chemistry
Publishing Model: Open Access
Deadline: Feb 28, 2026
Experimental and computational methodology in structural biology
Publishing Model: Open Access
Deadline: Apr 30, 2026





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in