
Having spent my entire scientific career trying to squeeze out finer and finer details of the atomic and molecular electron density distributions using high-resolution single crystal X-ray diffraction, I was immediately intrigued when I first laid eyes on the 2011 Chem Sci paper by Rinehart and Long[1] entitled "Exploiting single-ion anisotropy in the design of f-element single-molecule magnets". In particular, I was captivated by their Figure 8, which showed how the different electronic states of lanthanides are recognized by the strongly varying physical shape of the valence electron density distribution. What caught my interest was that my lifelong interest in electron densities could find very important use as an experimental tool in studies of single molecule magnets, which is in many ways still an emerging field despite being first seen more than 25 years ago.
The essence of the Rinehart & Long paper was quickly condensed (by others) to convey a dichotomous classificiation of all lanthanide ions as either oblate or prolate, meaning simply that the shape of their valence electron density distribution in the most magnetic state (which is the one that we want to stabilize) is either oblate or prolate. The neat thing about this is that the synthetic chemist can then attempt to surround the lanthanide by a crystal field with complementary shape. This simple approach has led to many important breakthroughs, among these the dysprosocenium which boasts an amazing blocking temperature. Nevertheless, their model was based entirely on theory and it was never experimentally verified. Now, this is exactly what X-ray diffraction has allowed us to do.
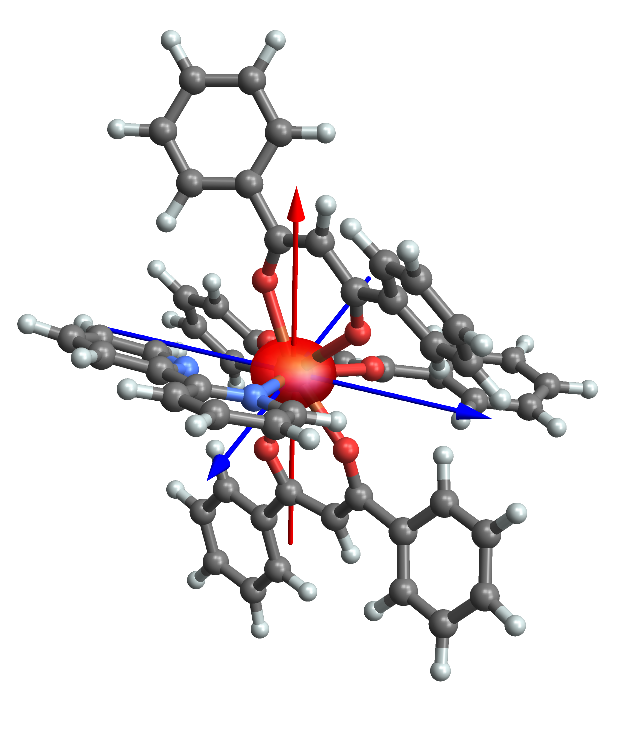
Fast forward past numerous futile attempts on obtaining data of sufficient accuracy to allow a detailed description of the valence density, we finally managed to obtain fine crystalline specimens of both known polymorphs of a classical dysprosium coordination complex with bipyridine and three β-diketonates. Obviously, this is not your everyday bachelor's project to salvage experimental information at this level of detail, and it requires a combination of highly intense X-ray sources only available at large synchrotron facilities, temperatures near the boiling point of Helium, and the most advanced X-ray detectors. But, 5 years after project kick-off we now have the first experimental images of what an oblate ion actually looks like! And it is not as oblate as we would have thought. As an unexpected bonus, we have actually been able to derive an approximate wavefunction composition of this molecule.
Obviously, the example in our paper is a single shot at one particular example, and we are obviously actively pursuing other, better single molecule magnets and exposing them to similar studies. We have justified hopes that we will have to wait less than five years for the next successful experiment, and the reason we believe this will be possible is that we have been involved in the testing of novel detectors and seen how exceptionally accurate and noise-free these are. So we certainly expect to be able to provide more examples in the future!
All this — and more — may be found in our recent paper.
[1] Rinehart, J. D. & Long, J. R. Exploiting single-ion anisotropy in the design of f-element single-molecule magnets. Chem. Sci. 2, 2078–2085 (2011).
Follow the Topic
-
Nature Chemistry

A monthly journal dedicated to publishing high-quality papers that describe the most significant and cutting-edge research in all areas of chemistry, reflecting the traditional core subjects of analytical, inorganic, organic and physical chemistry.





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in