What links structural inequality, neurodegeneration and epigenetics?
Published in Social Sciences, Neuroscience, and Genetics & Genomics
While aging is universal, the trajectory of cognitive decline or resilience is far from uniform and deeply influenced by the socioeconomic environments we navigate. The interplay between nurture (environmental and social exposome factors) and nature (genetic predisposition) plays a key (and differential) role in the development of neurodegenerative diseases like Alzheimer’s disease (AD) and frontotemporal lobar degeneration (FTLD). Recent findings from our study, published in Nature Aging, suggest once more that natural and social factors are more intertwined than we think.
Alzheimer’s vs. frontotemporal dementia in Latin America
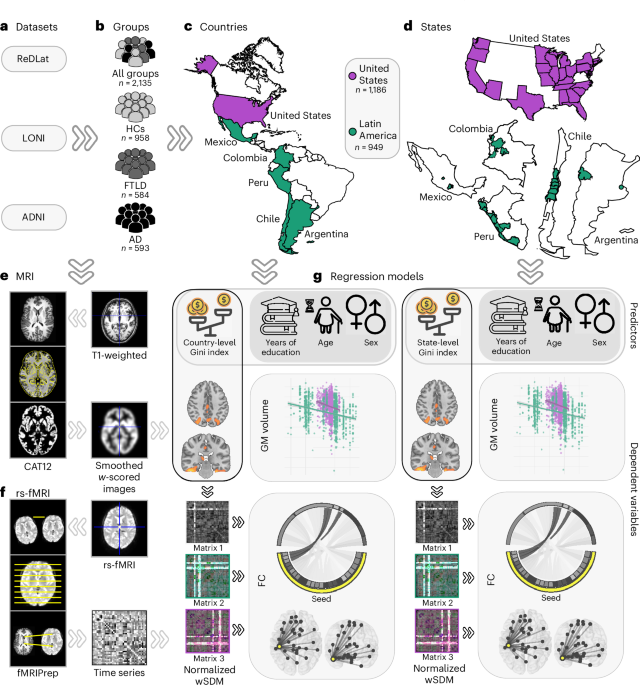
Our work examined 2,135 individuals across the Americas, showing that greater structural socioeconomic inequality, measured by the Gini index, is correlated with reduced brain volume and connectivity, particularly in Latin American countries. This suggests that the region's unique socioeconomic contexts, characterized by disparities in income, education, and access to resources, have a biological hallmark. One of our most compelling findings is the differential impact of structural inequality on brain health in Latin American individuals with AD compared to those with FTLD.
For patients with AD, higher levels of inequality were associated with significant reductions in brain volume and disrupted connectivity, particularly in brain areas critical for memory and cognition. These effects underscore their heightened vulnerability to socioeconomic stressors. In contrast, patients with FTLD exhibited a less marked association between inequality and brain structure or connectivity. This distinction aligns with the idea that environmental factors have a more substantial role in the development and progression of AD compared to FTLD, where genetic predisposition may have greater influence. Altogether, these findings propose a differential interplay between nurture and nature factors in shaping the brain’s aging trajectory across dementia phenotypes.
Epigenetics in neurodegeneration
Epigenetics—the study of changes in gene expression that do not alter the underlying DNA sequence—offers a robust framework for understanding how nurture exposome factors like socioeconomic inequality influence brain health. One key mechanism is DNA methylation, where environmental exposures leave a biological imprint by adding chemical groups to DNA, altering gene expression without changing the genetic code. These changes can be triggered by chronic stress, poor nutrition, or lack of access to healthcare, potentially accelerating neurodegeneration. In populations like those in Latin America, where socioeconomic disparities are profound, epigenetic mechanisms may play a crucial role in mediating the effects of inequality on brain structure and function. Incorporating epigenetic analyses into future research will be essential to untangling the complex interplay of nature and nurture in neurodegenerative diseases.
The importance of studying underrepresented settings
One of the most striking implications of our research is the opportunity to bridge neuroscience and social justice. Demonstrating that socioeconomic disparities through epigenetic pathways manifest as measurable changes in the brain underscores the urgent need for policies tailored to neurodegenerative diseases. These policies should focus on mitigating the impact of the exposome—the cumulative environmental influences on health—which is particularly strong in diseases like Alzheimer’s. By addressing inequalities through equitable education, improved healthcare access, and enhanced living conditions, we can potentially reduce the risk and delay the onset of such conditions. This is especially critical in Latin America, where the prevalence of Alzheimer’s disease is projected to increase exponentially in the coming years, making proactive and region-specific public health strategies essential to curb this growing burden. Also, the region’s extraordinary genetic diversity offers a unique opportunity to study ancestry-related genetic variations that interact with environmental and socio-economic factors. For example, populations in Latin America experience distinct combinations of indigenous, European, and African genetic contributions, which may influence susceptibility to diseases and the way epigenetic mechanisms like DNA methylation respond to chronic stress, poor nutrition, or environmental pollution. This interaction provides an ideal framework to explore how genetic predispositions and epigenetic modifications mediate the effects of structural inequality on brain health, highlighting pathways that might differ from those in more homogeneous populations.
A call to action
Our research is more than an academic endeavor; it is a call to action. As global citizens, we must advocate for systemic changes that foster equality in health. Our findings are a step forward in understanding the biological embedding of inequality. Still, they highlight the work yet to create a world where everyone can age with dignity and cognitive vitality.
The poster figure was created using GPT4 under the author's supervision.
Follow the Topic
-
Nature Aging

This journal’s mission is to provide a unique multidisciplinary, unifying and highly visible publishing platform for the aging-research community, with studies that cover the biology of aging and longevity.



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in