What´s in blood ? EN-RAGE enters the stage
Published in Neuroscience
Recent studies using large patient cohorts have high lightened the importance of specific genes and altered signaling networks in the brain as contributing factors for disorders like schizophrenia (1-3). Immune cells and modulators like cytokines are thereby thought to play a role during the acute phase of neuropsychiatric disorders (4), but what can trigger the diseases is still largely unknown. Can blood has something to do with it?
In our recent paper in Translational Psychiatry (5), we investigated the immune profiles in blood of a cohort of first episode acute psychosis (FEP) patients in comparison with age-matched healthy controls. In this study, we used the multiplex proximity extension assay-technique to determine the levels of up to 92 immune markers in blood plasma from 60 drug-naïve FEP patients and 50 controls. The results obtained were further evaluated by using different test and multivariate data analyses revealing statistical significant changes in 11 plasma proteins in FEP patients compared with controls. Among the proteins altered were cytokines like IL-6, the chemokine 23 as well as some others. The most significant change however was observed with the protein EN-RAGE also named S100-A12 and calgranulin C.
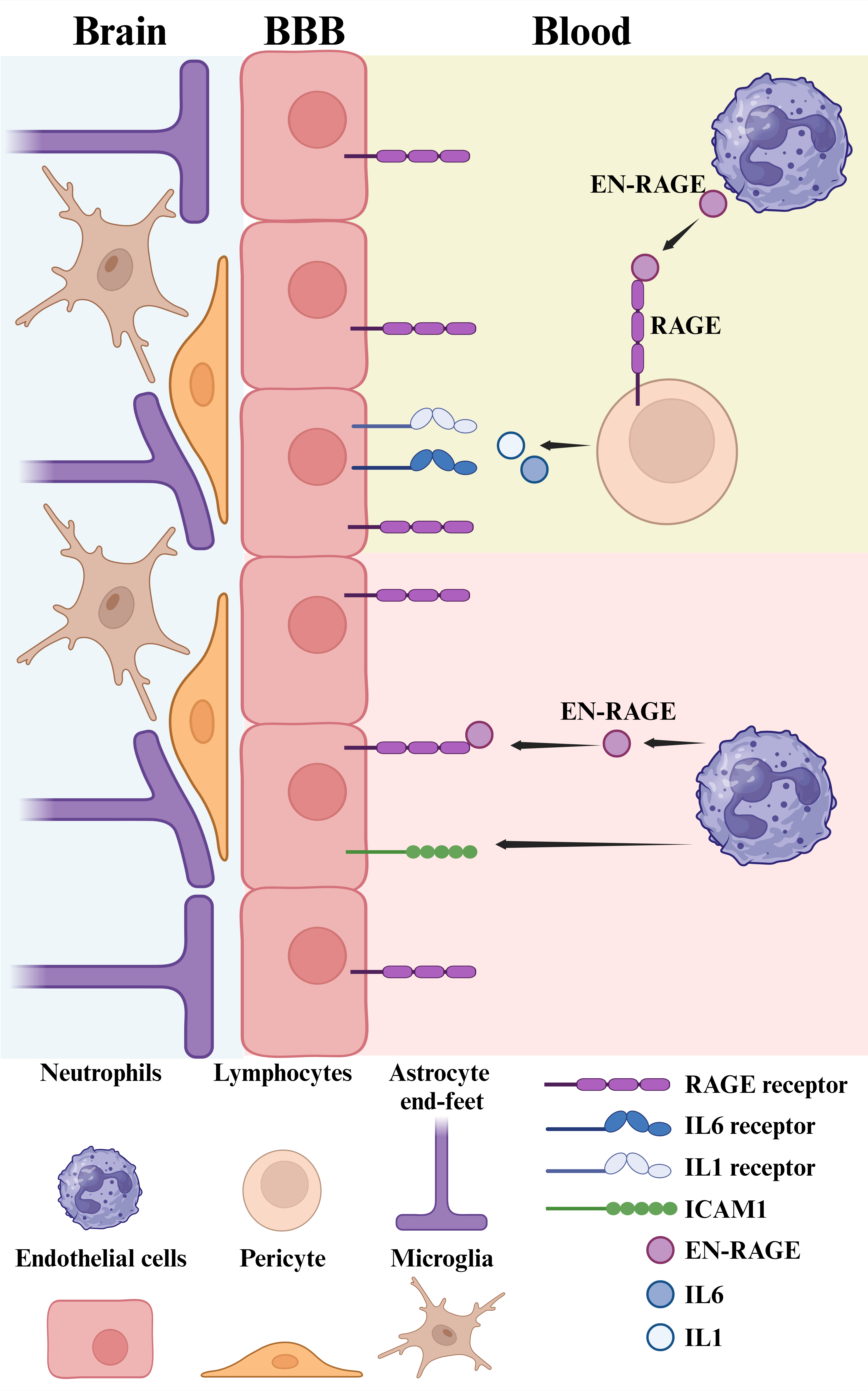
EN-RAGE is a member of the S100 family of calcium-binding proteins and is predominantly expressed by neutrophils, a type of white blood cells involved in immune responses and inflammation in the body (6,7). EN-RAGE binds to RAGE (receptor for advanced glycation end products) that is a multi-ligand receptor belonging to the immunoglobulin superfamily of transmembrane proteins. In addition to EN-RAGE, RAGE can bind also other ligands, such as AGEs (advanced glycation end products), HMGB1 (high-mobility group box-1), glycosaminoglycan and amyloid β peptides. The receptor RAGE is present on several cell types including lymphocytes and endothelial cells and the binding of EM-RAGE to RAGE elicits a cellular response that via the activation of the NFKappaB pathway leads to increased levels of pro-inflammatory cytokines (6,7). In the endothelium EN-RAGE/RAGE signaling can enhance the expression of cell adhesion molecules, such as the intracellular adhesion molecule (ICAM1), which then attracts more neutrophils to the vasculature in a positive feedback. This in turn may cause changes in the permeability of blood-brain barrier (BBB) with the possibility for entry of immune cells in the brain. In the brain parenchyma, the microglia, acting as local immunregulatory cells, express RAGE as do the astrocytes and neurons. Activation of RAGE in the brain has been linked to an enhanced neuroinflammation, for example as studied in models of Alzheimer´s disease, but the roles and expression pattern of EN-RAGE in the brain have not been delineated. Figure 1 summarizes in a schematic way the known biological actions of the EN-RAGE in inflammation and at the level of BBB.
As can be seen EN-RAGE plays a crucial role in the orchestrating of the inflammation processes that may accompany the debut of an acute neuropsychiatric condition such as psychosis. In this sense, increased EN-RAGE produced by neutrophils can instigate a cascade of events leading to local inflammation with the possibility of spread to the brain parenchyma.
Previous studies have shown alterations in cytokines in various neuropsychological disorders (4). Cytokines are also known to stimulate each other in an intricate pattern of molecular interactions, However, there is a hierarchy among immune modulators according which upstream cytokines regulate downstream effectors. In this sense, EN-RAGE can be considered as one of the master regulators that can stimulate the downstream cascade of other cytokines including IL-6. In our studied we observed increases in both EN-RAGE and IL-6 levels in FEP patients compared with controls, but so far this is only a correlation and warrant further studies. Likewise, it is not known whether the elevation in plasma EN-RAGE is restricted to the earliest phase of disease and abating thereafter. Such information would be of value when considering EN-RAGE as a possible biomarker for neuropsychiatric disorders such as schizophrenia. Previous studies in patients with coronary heart disease, showed that EN-RAGE represents an important biomarker for the heart affliction (8). In this regard, it is interesting to note that there is an elevated cardiovascular risk found in schizophrenia and other severe mental disorders, but the biological reason for this has remained unclear (9).
In conclusion, we observed increases in the plasma of the immune regulator EN-RAGE in first episode patients with acute psychosis compared with healthy controls. EN-RAGE is expressed by neutrophils and as shown here can affect the immune response and the brain endothelium in different ways likely promoting the disease progress. We suggest that EN-RAGE expressed by neutrophils could constitute a novel, easy accessible, blood-born marker for acute psychosis. However further research on mechanisms with analyzes also of data from other patient cohorts will be helpful to understand the precise role EN-RAGE in neuropsychiatric diseases. The studies of EN-RAGE could also give valuable insights about immune pathways in brain diseases in general.
Our findings with EN-RAGE show that blood-born factors play a distinct role in the acute phase of the disease. The precise mechanisms involved and the roles of neutrophils in the process warrant further clarifications, but the result is an example of how brain and body are unified and react together in a disease context. And this is what many of us have instinctively always recognized.
Acknowledgements
We thank our colleagues, and all patients that made this study possible.
References
1.van der Meer D, Cheng W, Rokicki J, Fernandez-Cabello S, Shadrin A, Smeland OB, et al. Clustering Schizophrenia Genes by Their Temporal Expression Patterns Aids Functional Interpretation. Schizophr Bull. 2023 Oct 12: sbad140.
2.Liu D, Meyer D, Fennessy B, Feng C, Cheng E, Johnson JS, et al. Schizophrenia risk conferred by rare protein-truncating variants is conserved across diverse human populations. Nat Genet. 2023;55(3):369-376.
3.Smeland, O.B., Andreassen, O.A. Schizophrenia: genetic insights with clinical potential. Nat Rev Neurol. 2022; 18, 129–130.
4.Pillinger T, Osimo EF, Brugger S, Mondelli V, McCutcheon RA, Howes OD.Meta-analysis of Immune Parameters, Variability, and Assessment of Modal Distribution in Psychosis and Test of the Immune Subgroup Hypothesis. Schizophr Bull.2019; 45(5): 1120–1133.
5.Korhonen L, Paul ER, Wåhlén K, Haring L, Vasar E, Vaheri A, Lindholm D. Multivariate analyses of immune markers reveal increases in plasma EN-RAGE in first-episode psychosis patients. Transl Psychiatry 2023; doi 10.1038/s41398-023-02627-8.
6.Hudson BI, Lippman ME. Targeting RAGE Signaling in Inflammatory Disease. Annu Rev Med. 2018;69:349-364.
7.Dong H, Zhang Y, Huang Y, Deng H. Pathophysiology of RAGE in inflammatory diseases.Front Immunol. 2022;13:931473.
8.Ligthart S, Sedaghat S, Ikram MA, Hofman A, Franco OH, Dehghan A. EN-RAGE: a novel inflammatory marker for incident coronary heart disease. Arterioscler Thromb Vasc Biol. 2014 ;34(12):2695-9.
9. Rossom RC, Hooker SA, O'Connor PJ, Crain AL, Sperl-Hillen JM. Cardiovascular Risk for Patients With and Without Schizophrenia, Schizoaffective Disorder, or Bipolar Disorder. J Am Heart Assoc. 2022;11(6):e021444.
Figure 1.
EN-RAGE is produced by neutrophils and acts on adjacent lymphocytes via its receptor RAGE. This causes an increase in the synthesis and release of cytokines such as IL-6 and IL-1. These in turn act as pro-inflammatory agents in the body and on the brain endothelium as part of the blood-brain barrier (BBB). The endothelial cells express RAGE and respond to EN-RAGE by increases in adhesion molecules, such as ICAM-1 that leads to an enhanced recruitment of neutrophils and their transmigration into brain tissue. BBB can also become more permeable to other brain cells such as lymphocytes further aggravating the inflammation with activation of microglial cells. The schema shown depicts an intricate web of cellular interactions involving EN-RAGE that will require further investigations. The figure is a courtesy by Dr. Vignesh Srinivasan.
Follow the Topic
-
Translational Psychiatry

This journal focuses on papers that directly study psychiatric disorders and bring new discovery into clinical practice.
Your space to connect: The Psychedelics Hub
A new Communities’ space to connect, collaborate, and explore research on Psychotherapy, Clinical Psychology, and Neuroscience!
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
Moving towards mechanism, causality and novel therapeutic interventions in translational psychiatry: focus on the microbiome-gut-brain axis
Publishing Model: Open Access
Deadline: May 19, 2026
From mechanism to intervention: translational psychiatry of childhood maltreatment
Publishing Model: Open Access
Deadline: Feb 28, 2026






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in