Whole brain profiling of cocaine and sucrose processing in mice
Published in Neuroscience

Explore the Research

Whole-brain tracking of cocaine and sugar rewards processing - Translational Psychiatry
Translational Psychiatry - Whole-brain tracking of cocaine and sugar rewards processing
Global brain activation after pharmacological and natural reward exposure
Addiction is a miserable disease that has catastrophic consequences on societies and individuals’ lives. Especially resilient to treatment are addictions to drugs of abuse, like alcohol, opioids, or cocaine. Often it is hypothesized, that liking these substances relies on neuronal circuitry usually processing pleasant, non-addictive experiences driven by naturally-available rewards. Thus, in our article, we describe our efforts for understanding whether the information about addictive substances is processed by similar brain regions as natural rewards.
To address this question, mice were exposed to either cocaine or sucrose solution in an acute (single) or prolonged (7 consecutive days) manner. In the brains of these mice, we studied the expression of c-fos, which in neurons is a gene corresponding to elevated neuronal activity. To holistically examine the elevated c-fos level in the central nervous system we used a unique technique for optical brain clearing and immunolabelling, called iDisco+. In this method, the tissue become transparent to light, which allows for the imaging of the whole intact rodent brain. The images of our samples with marked c-fos expression were analyzed using ClearMap software, which has the capacity to automatically count all c-Fos-positive cells in the brain. We then aligned the c-Fos positive cells with the standardized atlas of the mouse brain, provided by The Allen Institute, and as an outcome, we determined the activity level of almost 400 brain structures of mice exposed to either sucrose or cocaine.
In general, we found cell activation in distant parts of the mouse brain, which was far more extended beyond the classically considered brain-reward network. We found parts of the brain that were highly activated only by cocaine (e.g. perirhinal cortex) or sugar (e.g. lateral septal nucleus) or by both types of rewards (e.g., nucleus accumbens). Altogether, a single sucrose exposure resulted in a higher number of activated structures compared to repeated natural reward treatment. In contrast, the acute and prolonged cocaine treatment caused the opposite pattern of brain activation. Here, 7 days of exposure elevated c-fos expression levels in more brain regions than a single dose of cocaine. This prolonged cocaine exposure caused excessive brain activation, which covered most of the studied structures.
Next, we examined how cocaine and sucrose exposure alter the global organization pattern of neuronal populations. Thus, we performed the functional connectivity analysis to identify correlated structures, which share the most and least similar pattern of c-fos expression. This approach allowed us to determine the subnetworks, called modules, which unitedly process rewards. We found that acute exposure to both rewards increases the modularity of the brain, which may indicate global neuronal reorganization. This increased modularity stabilizes during prolonged exposure to sucrose, but not cocaine. These findings go along with the hypothesis that natural rewards are stimuli, in which novelty is an important component. On the other hand, drugs of abuse seem to be a “better than expected” reward, which more broadly engages the brain after repeated usage.
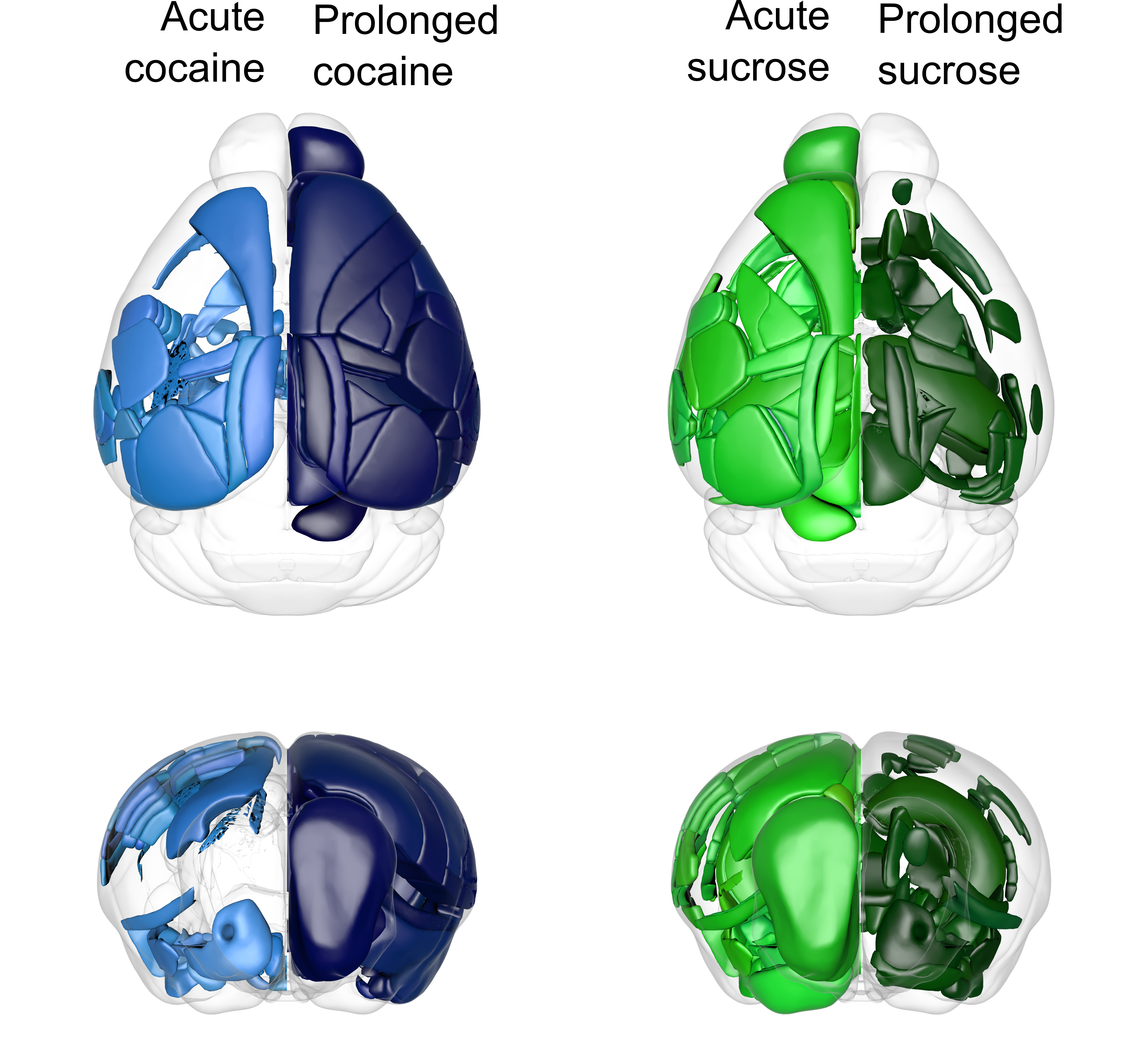
Fig. 1: Patterns of mouse brain activation by cocaine (blue) or sucrose (green) exposure. Light colors correspond to acute exposure and dark to prolonged one.
Are cocaine and sucrose processed differently at the molecular level?
Few brain structures were activated by both sucrose and cocaine exposure and one of the most activated ones was the nucleus accumbens (ACB). This deeply located structure has a well-documented role in reward processing and is often pointed out as the core of drug-liking and developing an addiction. ACB has also well understood anatomical structure and is composed of dopamine-sensitive cells, which express one of two types of dopamine receptors: D1 or D2. Thus, ACB seemed like a perfect structure for molecular-level comparison of the processing of cocaine- and sucrose-related experiences.
Here, transgenic mice expressing endogenous fluorescence in either D1-type or D2-type neurons were exposed to sucrose or cocaine for 7 days. On slices from their brains, we performed a series of electrophysiology experiments to study plastic changes, which are cellular adaptations indicating for remodeling of neural circuitry. We found that both sucrose and cocaine engage D1 and D2 cells in ACB, by creating silent synapses, which are immature connections between neurons. Moreover, we found that both rewards similarly increase the excitatory drive onto D1-type neurons and weaken it in D2-type cells. This molecular-level analysis unraveled that in the ACB, cocaine engages neural circuitry physiologically processing natural rewards. 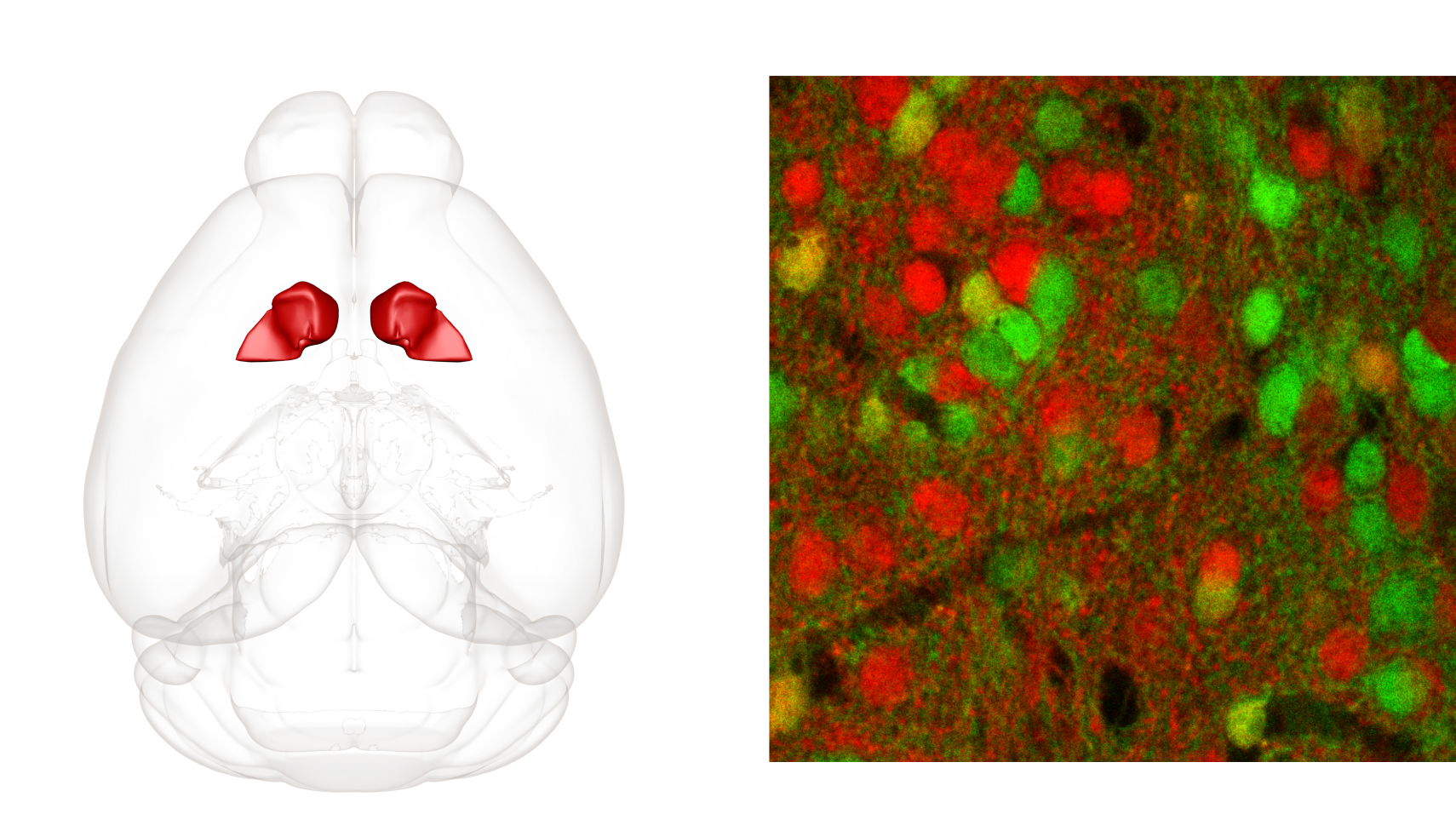
Fig. 2: Localization of the nucleus accumbens in a mouse brain (left) and dopamine-sensitive neurons in a mouse nucleus accumbens (right). The nucleus accumbens is mainly composed of neurons expressing either D1-type (red) or D2-type (green) dopamine receptors and these are highly non-overlapping populations.
Figures were created using the Scalable Brain Atlas
Follow the Topic
-
Translational Psychiatry

This journal focuses on papers that directly study psychiatric disorders and bring new discovery into clinical practice.
Your space to connect: The Psychedelics Hub
A new Communities’ space to connect, collaborate, and explore research on Psychotherapy, Clinical Psychology, and Neuroscience!
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
Moving towards mechanism, causality and novel therapeutic interventions in translational psychiatry: focus on the microbiome-gut-brain axis
Publishing Model: Open Access
Deadline: May 19, 2026
Precision medicine approaches in psychiatry
Publishing Model: Open Access
Deadline: May 13, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in