World AMR Awareness week: Guidelines for responsible antimicrobial use in food-producing animals
Published in Microbiology, Public Health, and Zoology & Veterinary Science

The problem of antimicrobial resistance
Antimicrobial resistance (AMR) is a major global health concern, that weakens our ability to treat infections and increases the risk of disease spread, severe illness, disability, and death. AMR knows no borders and deeply affects the health of people, animals, and ecosystems. Antimicrobials include antibiotics, antifungals, antiparasitics, and antivirals and their inappropriate use in human healthcare, veterinary medicine, and agriculture accelerates resistance which can spread through water, soil, and food chain [1].
Under the One Health approach – which recognizes the link and interdependence between the health of humans, animals, and the environment – international efforts are being made to safeguard the effectiveness of treatments and ensure a sustainable future. Among these efforts are the design and implementation of guidelines for the responsible use of antimicrobials in food-producing animals [2,3].
What did the authors do?
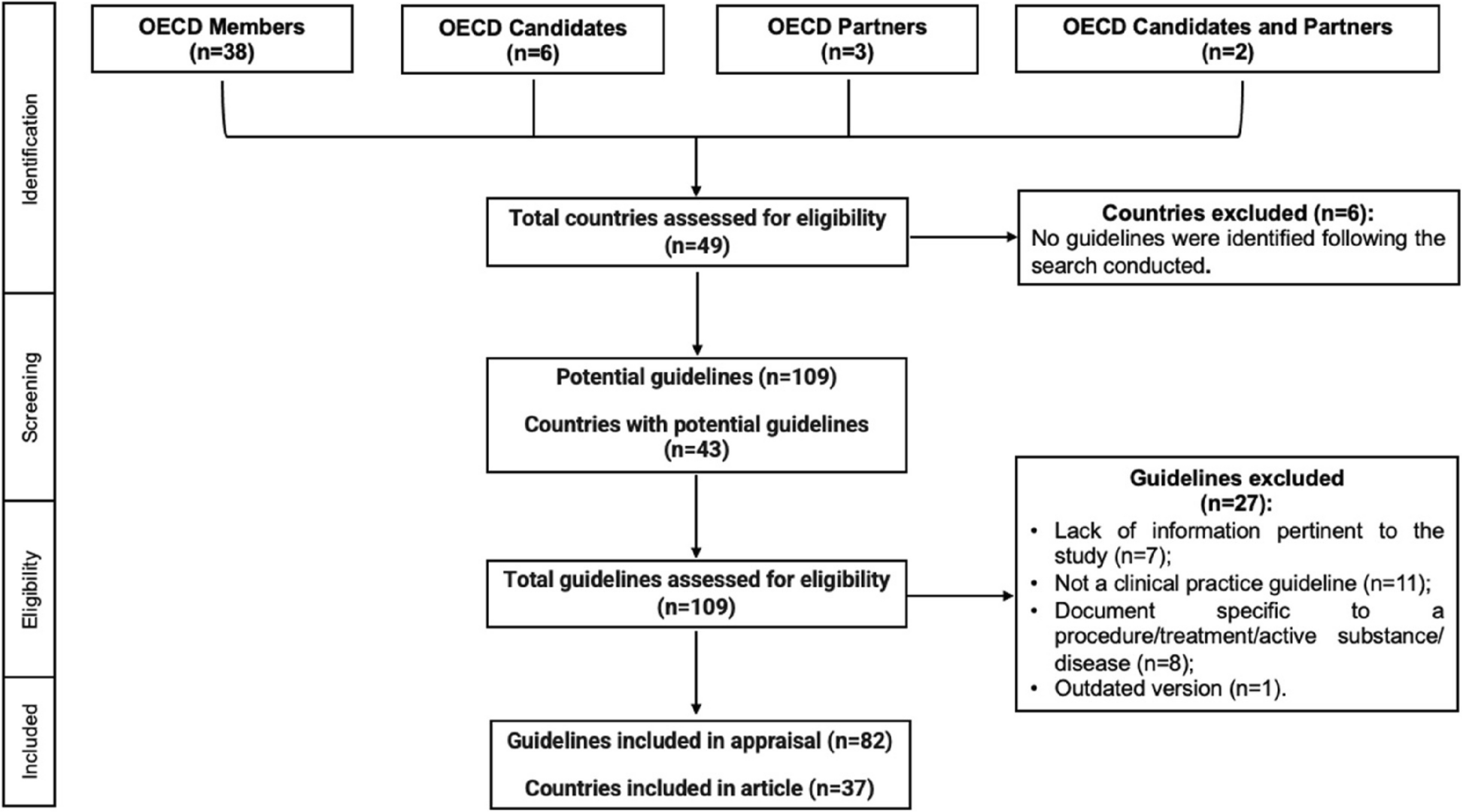
This systematic review combines comprehensive searches and critical assessment of published guidelines on antimicrobial use in livestock (bovine, caprine, equine, ovine, and swine) across 49 OECD countries (members, partners, and candidates). The authors collected guidelines from official government websites, veterinary organizations, and international repositories, ensuring a broad and inclusive dataset. To evaluate the quality and consistency of these guidelines, the authors used the AGREE II instrument, a standardized tool that assesses multiple aspects of guideline development, such as clarity, rigor, and stakeholder involvement.
The authors aimed to:
- Identify guidelines’ main characteristics, strengths, and weaknesses.
- Assess guidelines’ compliance with international recommendations – published by the World Health Organization (WHO), the Food and Agriculture Organization (FAO), and the World Organisation for Animal Health (WOAH).
What did the authors find?
Of the 49 OECD countries, 37 of them presented a total of 82 guidelines for responsible antimicrobial use in food-producing animals (Figure 1), with guidelines targeting conditions in bovine and swine species being the most represented. The highest number of published guidelines was observed between 2017-2020. Also, the number of guidelines increased significantly after 2016, reflecting global efforts aligned with international initiatives, like the WHO's Global Action Plan on AMR [4].
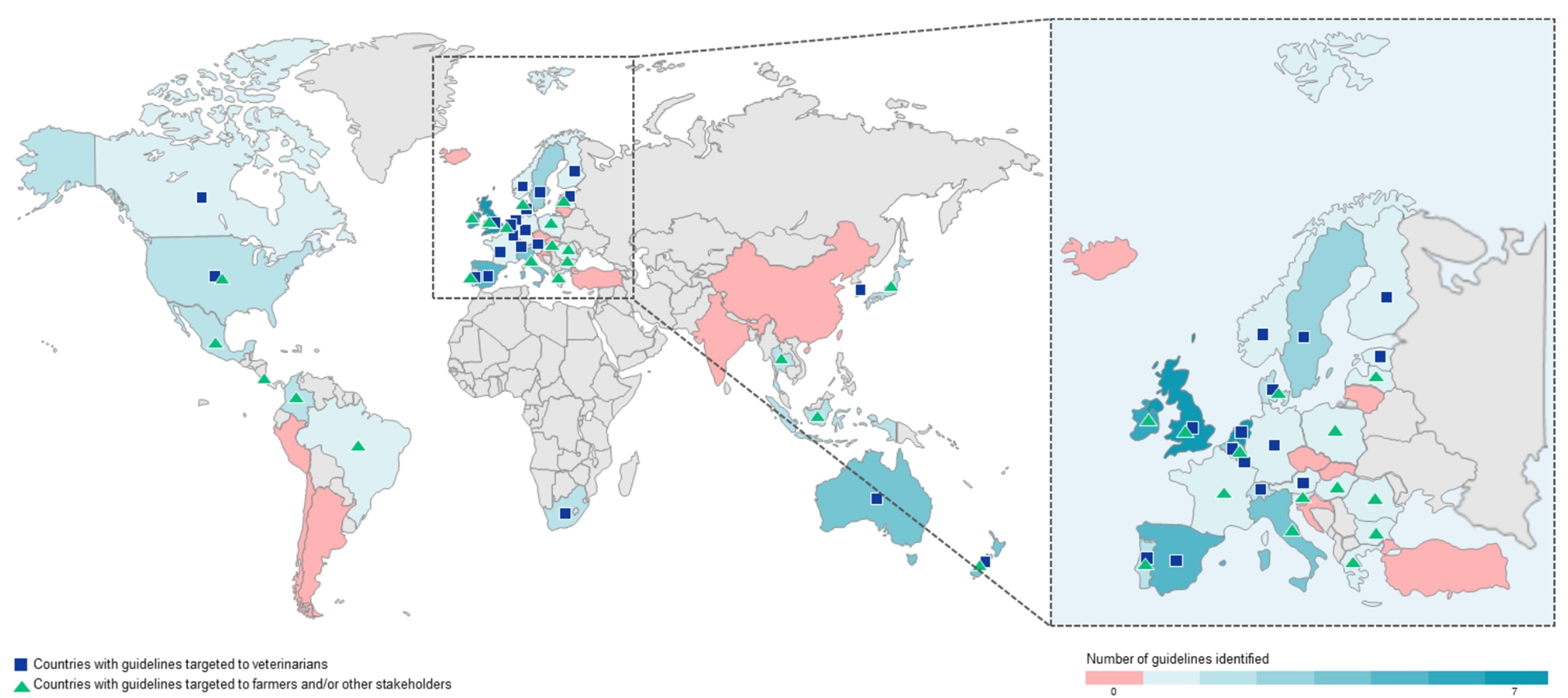
Figure 1. World map showing the number of guidelines across OECD countries and target audience.
Overall, countries recognize AMR as a major threat and are working diligently to promote responsible antimicrobial use in livestock. Nevertheless, some countries need further support for effective implementation of guidelines to strengthen global efforts against AMR.
What are the limitations of this study?
Despite providing valuable insights, this study relied on publicly available guidelines from official sources, which may not reflect actual practices or enforcement levels within countries. Moreover, the real-life impact of these antimicrobial use guidelines should be analyzed through data on antimicrobial use and/or sales and AMR prevalence.
What are the implications?
This systematic review helps identify gaps that need to be addressed for better antimicrobial use and more sustainable agriculture practices. This work can inform policymakers and international agencies in designing more robust and evidence-based frameworks adapted to diverse national contexts and available resources. Barriers to implementation of guidelines should be addressed, international knowledge exchanged, and collaboration encouraged to fight AMR within the One Health approach.
References
[1] WHO. 10 Global health issues to track in 2021. 2020. https://www.who.int/news-room/spotlight/10-global-health-issues-to-track-in-2021. Accessed 15 Oct 2025.
[2] WHO. Fact sheet - One Health. 2023. https://www.who.int/news-room/fact-sheets/detail/one-health. Accessed 15 Oct 2025.
[3] WHO. Global call to action to address antimicrobial resistance: An appeal for keeping our medicines against infectious diseases effective to save lives. 2025. Available at: https://www.who.int/publications/m/item/global-call-to-action-to-address-antimicrobial-resistance. Accessed 15 Oct 2025.
[4] WHO. Global action plan on antimicrobial resistance. 2016. Available at: https://www.who.int/publications/i/item/9789241509763. Accessed 15 Oct 2025.
Follow the Topic
-
One Health Outlook

This journal is a new open access journal published by BMC in collaboration with the Global One Health Community.
Related Collections
With Collections, you can get published faster and increase your visibility.
One Health aspects of acute respiratory virus infections of humans and animals
The growing understanding of the intricate connections between human, animal, and environmental health highlights the critical need for a One Health approach in addressing emerging and re-emerging respiratory viral infections. Zoonotic viruses such as influenza, SARS-CoV-2 (COVID-19), and other coronaviruses, as well as common respiratory pathogens like respiratory syncytial virus (RSV), human metapneumovirus (HMPV), and adenoviruses, continue to pose significant public health and veterinary challenges worldwide.
We invite submissions of original research articles, reviews, and case studies that explore various One Health dimensions of acute respiratory virus infections affecting humans and animals. Topics of interest include, but are not limited to- Zoonotic transmission and interspecies spread, Epidemiology and surveillance, Pathogenesis and immune responses, Diagnostic advancements, Prevention and control strategies, Environmental and ecological factors, and Public health and veterinary collaboration.
All submissions in this collection undergo the journal’s standard peer review process. Similarly, all manuscripts authored by a Guest Editor(s) will be handled by the Editor-in-Chief. As an open access publication, this journal levies an article processing fee (details here). We recognize that many key stakeholders may not have access to such resources and are committed to supporting participation in this issue wherever resources are a barrier. For more information about what support may be available, please visit OA funding and support, or email OAfundingpolicy@springernature.com or the Editor-in-Chief.
Publishing Model: Open Access
Deadline: Mar 24, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in