World Tuberculosis Day
Published in Sustainability and Public Health

What is TB and how smoking impacts TB
Tuberculosis (TB) is a bacterial infection spread through respiratory droplets dispersed into the air, when someone with TB coughs and sneezes. Smoking increases the chances of contracting TB infection and TB disease. TB patients who smoke are more likely to experience severe forms of clinical presentation, poor response to treatment both bacteriologically and clinically, and have higher rates of treatment failure compared to non-smokers. The strong association between smoking and TB has led to increasing recognition for more evidence-based approaches to smoking cessation for TB patients.
Potential for digital and mHealth solutions for TB patients who smoke
Our previous randomised controlled trials (RCT) in Bangladesh and Pakistan provided strong evidence to support face-to-face behavioural interventions in achieving high quit rates. Despite the known benefits of integrating behavioural interventions into TB treatment, no country with high burden of TB has yet been able to consistently provide in-person counselling to help patients with TB quite smoking due to challenges related to costs, reach and sustainability. To address the challenges of scaling up face-to-face interventions, WHO have developed mTB-Tobacco to help TB patients quit smoking by sending motivational and informative short message messages (SMS) message to their phones. Although mHealth interventions through text messages are cheaper to deliver than in-person sessions, there is still uncertainty regarding whether they can achieve the same level of effectiveness. Further research is needed to determine their true value.
Quit4TB Trial
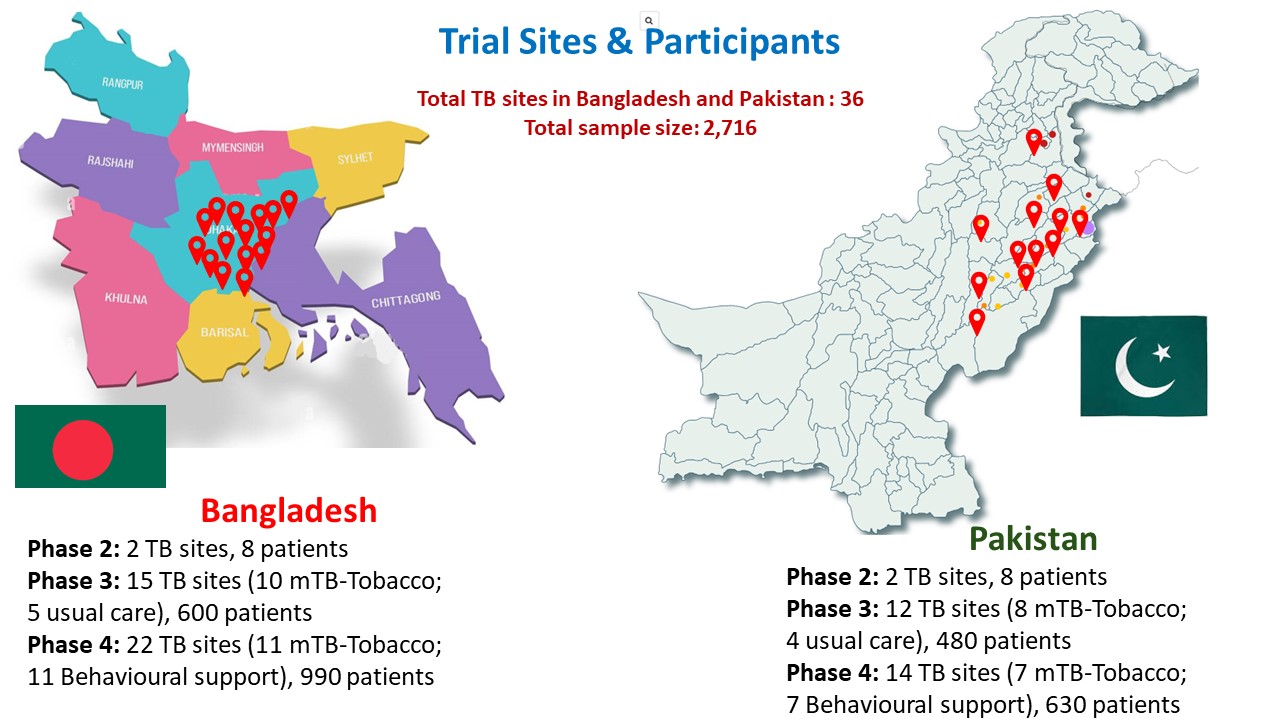
Our aim was to assess the effectiveness and cost-effectiveness of mTB-Tobacco in smoking cessation in TB patients who smoke daily in Bangladesh and Pakistan. We also assessed the effectiveness and cost effectiveness of mTB-Tobacco in enhancing TB treatment adherence and improving clinical outcomes. In this multi-center, cluster randomised, controlled trial, SMS texts were sent to patients with TB to encourage behaviour change and provide motivational and informative messages. A total of 2,716 smokers with TB were recruited in this study.
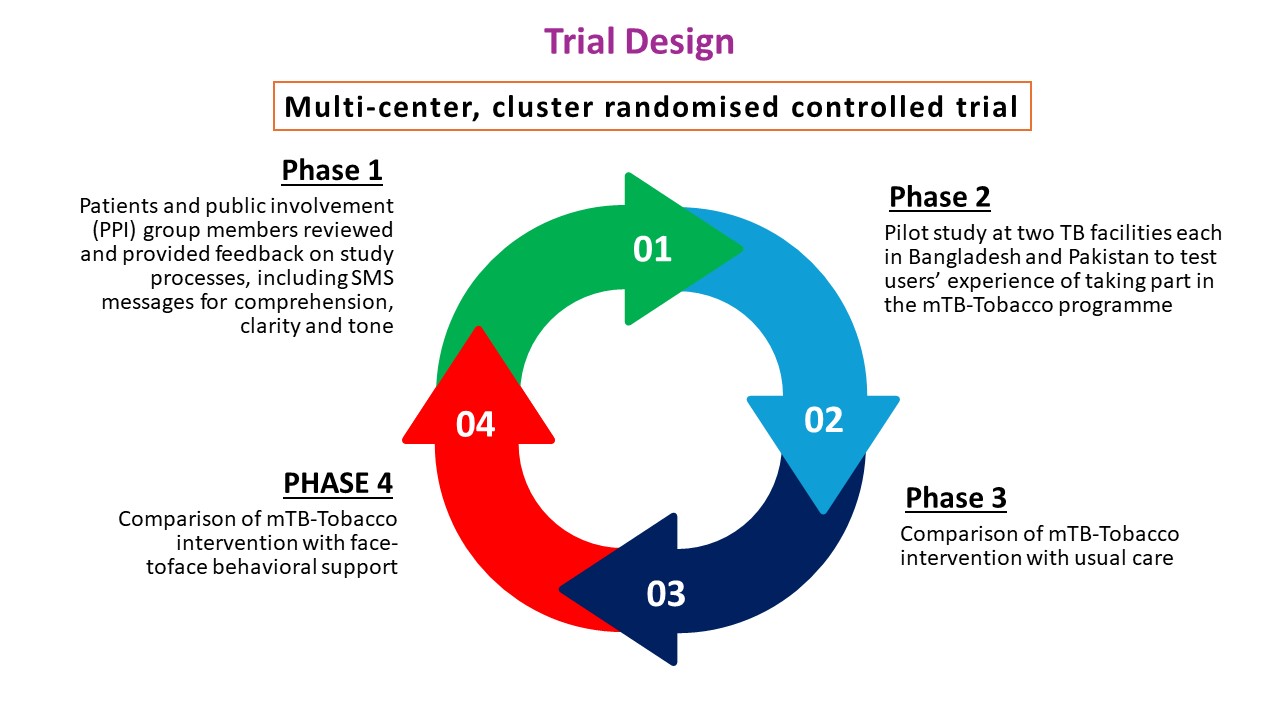
Phase 1 Patient and Public Involvement (PPI) activities
Phase 1 of this study involved consultation with the PPI members about the study processes. PPI members helped review and provide feedback on the participant facing materials including SMS messages for comprehension, clarity, and tone. In Bangladesh, the PPI members comprised of one manager, five DOTS (directly observed treatment, short-course) workers and five TB patients. In Pakistan, the eight members comprised of two TB patients, two family members, three general public members, and one non-medical TB staff members. PPI members were briefed about the study and their roles in this study. Translated versions of the SMS messages were shared with PPI members. SMS messages that were considered as unclear or inappropriate to the PPI members were rewritten.
Phase 2 pilot study
A total of 16 participants were recruited. The pilot evaluated using two sets of data: users’ self-reported experiences and their real-time engagement with the programme. Users’ experiences were collected through face-to-face/phone-based interactions. Key questions focused on users’ experience of taking part in the mTB-Tobacco programme; the clarity, quantity, timing and frequency of messages; what was good about the programme and what was not; completion or non-completion the programme; and any effect on their attitudes or target behaviour(s).
Phase 3 superiority trial
We compared mTB-Tobacco (intervention) with usual care (control). Each participant in the intervention group received a total of 134 SMS messages over a period of 6 months. In the first two months, the frequency of messages was 4 to 5 messages per day, in next two month the frequency reduced to 1 to 2 messages per day and in the last two months only 1 message was sent per week. In addition to mHealth smoking cessation package, the intervention group received education leaflets from TB health professionals. Each participant in the control group received the same patient education leaflets from TB health professionals. The leaflets contain information on the harmful effects of tobacco and advice on stopping its use.
Phase 4 non-inferiority trial
We compare mTB-Tobacco with face-to-face behavioural support. Participants in face-to-face behavioural support will receive two face-to-face sessions delivered at day 0 and day 5 (+2) and which last 10 and 5 minutes respectively. The sessions will be structured using an educational flipbook; the session on day 0 will be aimed at encouraging tobacco users to see themselves as non-users and set a plan for a quit date five days later; with a session on the quit date (day 5) to review progress. Further encouragement and support (if needed) will be offered at a subsequent visit at week 5.
Engagement with patients, carers, communities, healthcare providers and government
During the implementation of the trial, the QUIT4TB trial study teams in Bangladesh and Pakistan engaged with TB patients, their caregivers, treatment providers, and authorities overseeing TB program operations at both grassroots and national levels. A stakeholder and community engagement team (formed by RESPIRE and the local study teams) involved various stakeholders from the beginning of the project and engaged with them throughout the project lifecycle. The engagement activities fostered effective relationships among stakeholders while sharing experiences, raising awareness about TB, and empowering them with knowledge related to TB treatment and trial ethics. To help bridge the gap between research, policy and practice, the local study teams presented the trial summary and shared preliminary findings with the policymakers, public health researchers and practitioners. Stakeholder engagement is an ongoing effort of this trial to foster strong relationships, trust and buy-in for future policy development and implementation.
Follow the Topic
-
ISRCTN: The UK’s Clinical Study Registry

A primary clinical trial registry recognised by WHO and ICMJE that accepts studies involving human subjects or populations with outcome measures assessing effects on human health and well-being, including studies in healthcare, social care, education, workplace safety and economic development.






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in