Zwitterionic “Implant Mate” enables long-term use of subcutaneous insulin infusion catheters and faster pharmaceutical absorption
Published in Bioengineering & Biotechnology
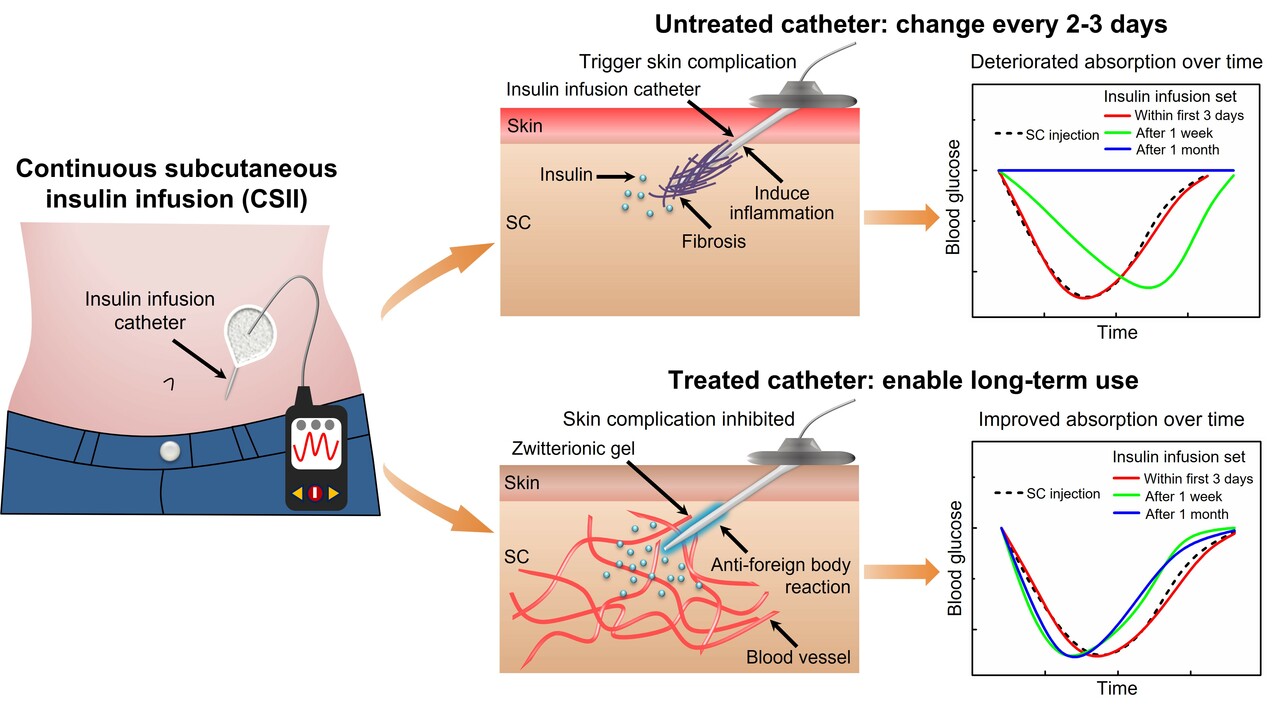
Subcutaneous (SC) infusion through indwelling catheters is commonly used throughout the hospital and at home for administering needed medications to provide appropriate symptom management. So far, SC catheters have been demonstrated to be effective for administering insulin, anticoagulants, granulocyte colony-stimulating factors, morphine, growth hormones, and other medications in patients. Compared to painful daily or multiple daily needle injections, an indwelling SC catheter could assist in drug delivery (either as a continuous infusion or for bolus doses) and decrease trauma, distress, and discomfort for patients. Continuous subcutaneous insulin infusion (CSII) through indwelling catheters is the most common application scenario and has become the standard insulin replacement therapy for type 1 diabetes management. Compared to multiple daily injections, CSII shows the convenience for fulfilling various insulin needs through the indwelling catheter and a series of therapeutic advantages, including improved glycemic control (lowered hemoglobin A1c, fasting, and postprandial blood glucose levels, and nocturnal hypoglycemia incidence), decreased insulin dosage requirement, and improved health-related quality of life. Currently, CSII has been adopted by more than one million patients with type 1 diabetes worldwide, and this therapeutic option has been extended to type 2 diabetes as recommended by many countries.
Despite various commercial catheters being available, the issues with CSII catheters have never been addressed, including skin irritation, high occlusion rates, and poor glycemic outcomes. Consequently, the current catheters still require changing and rotating their insertion site every 2-3 days when used in the SC tissue; the recommended duration of use never changed since 1983. This, however, creates inconvenience and high costs for pump users and can hardly match the wear time duration of continuous glucose monitors (CGMs), such as up to 14 days, for closed-loop integration. This has become the top concern among pump users. There is a strong need to develop a technology to address these issues and significantly extend the longevity and performance of current infusion catheters. Unfortunately, so far, nearly no strategy has demonstrated efficacy.
To address this issue, our lab developed an injectable zwitterionic cream gel, namely Implant Mate, that can be conveniently applied to modify CSII catheters, drastically extends the longevity of the catheters, and improves the rate of insulin absorption. Our major findings include: (1) The Implant Mate could perform an anti-inflammatory role and remarkably suppress skin complications and thus was able to fully resolve the catheter-induced skin complications, one of the primary reasons preventing patients from long-term wearing of the catheters. (2) The Implant Mate drastically enhanced the longevity of CSII catheters for more than 6 months without occlusion in three different but increasingly challenging mouse models. However, the untreated catheter elicited intense foreign body reactions, impairing insulin absorption and occlusion over time, the issues frequently observed clinically when catheter wear time was increased. (3) The Implant Mate resulted in faster insulin absorption through the implanted catheter, even when compared with the conventional syringe-based SC injection. This benefit results from the zwitterionic gel, which could promote the formation of new blood vessels and maintain their high-density level surrounding the SC-implanted catheter, leading to accelerated mass transport between the body and the catheter. (4) The performance of the Implant Mate was further evaluated in a diabetic large animal model. The pig study suggested that the Implant Mate treatment can drastically increase the longevity of the implanted catheters from 2 days to 13 days under practical pump therapy conditions. In particular, the degradable zwitterionic gel can be gradually degraded and absorbed by the pig within 30 days without needing to be removed by additional surgery.
For the first time, our injectable zwitterionic Implant Mate addresses the clinical problem associated with CSII catheters that has no previous solution. It addressed the major causes that clinically prevent the long-term wearing of CSII catheters, resulting in a significant leap in the longevity of inserted catheters. The drastically extended wear duration (from 2 to 13 days in diabetic pigs) will allow matching with the dwelling duration of current implantable CGMs for closed-loop integration, a central unsolved pain point for diabetes management.
Faster insulin absorption has been a key focus in the diabetic therapeutics development for improved glycemic control. Current efforts are limited to insulin modification, such as developing insulin analogs absorbed faster than native insulin. The R&D is usually costly and subject to high regulatory risk. Our Implant Mate technology resulted in faster insulin absorption, representing a new strategy for rapid-acting insulin therapy.
The Implant Mate application differs from the traditional coating formation strategy involving complex chemistries or processes. It is subcutaneously injected before the tip of the catheter is inserted into it and should generally apply to CSII catheters. It applies to other implantable devices (the reason called “Implant Mate”), particularly when tissue reactions to the device surface are of concern.
As my first project in the Cao lab (http://caoresearch.com/), this work spanned approximately six years from its beginning to the final acceptance of the article. It is a long and challenging journey, but we take immense pride that this technology provides a novel strategy to address an urgent clinical problem for the first time and show high performance and clinical translatability. It transforms the current knowledge and methodologies in addressing the vexing problem of implanted catheters and devices. It potentially impacts the performance of all current off-the-shelf commercial catheters.
Follow the Topic
-
Nature Biomedical Engineering

This journal aspires to become the most prominent publishing venue in biomedical engineering by bringing together the most important advances in the discipline, enhancing their visibility, and providing overviews of the state of the art in each field.




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in