Exploring the Fascinating Dynamics of Supercooled Water Droplets: From Classroom to Publication
Published in Physics
Introduction
The dynamics of phase change in water play a crucial role in energy-related and aerospace processes. Our research focuses on understanding the behavior of supercooled water droplets on superhydrophobic surfaces, where they exhibit unexpected jumping motions upon freezing. This unique phenomenon is driven by a remarkable force known as vaporization momentum, which occurs when the droplet's surface vaporizes in a low-pressure environment. This force is intensified by a process called recalescence, where the droplet quickly reheats upon freezing, leading to droplet compression and self-jumping. Through our study of the interplay between liquid-gas-solid phase changes and interactions with the solid surface, we have uncovered the underlying mechanisms behind the vaporization momentum and droplet dynamics. We have also found that the jumping velocity of the droplets is influenced by their size, with larger droplets typically exhibiting higher velocities. The direction of the jumping motion is governed by a process called nucleation, which involves the formation of tiny ice crystals. To categorize droplet behavior based on size, we have established a regime map that distinguishes self-jumping induced by vaporization momentum from other phenomena such as evaporative drying and overpressure-initiated levitation caused by depressurization and vaporization. By understanding the role of supercooling and low-pressure-mediated phase change in fluid transport dynamics, we can develop more efficient strategies for anti-icing applications, enhance cooling technologies, and gain insights into climate-related freezing physics.
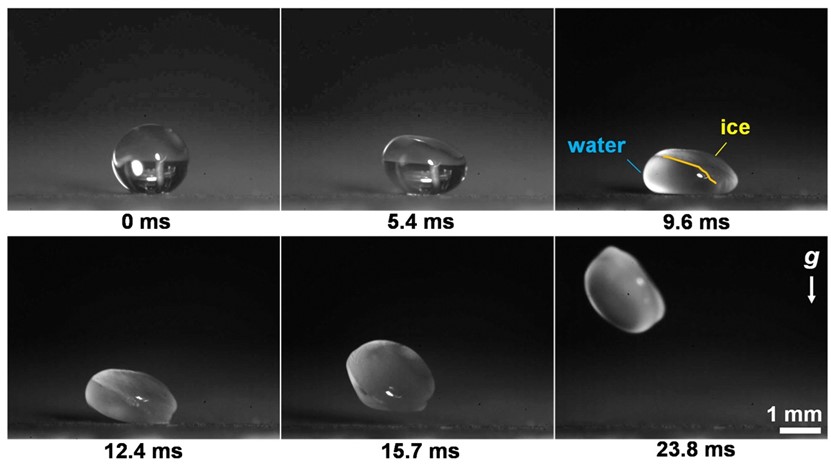
Figure 1. Time-lapse photographs showing the jumping droplet from a superhydrophobic surface as it freezes in a low-pressure environment (~100 Pa).
From Classroom to Laboratory
In the fall of 2021, my heat transfer class at Hong Kong University of Science and Technology (HKUST) sparked the interest of four undergraduate students (Sui Cheong Chan, Ying Lung Chan, Ngai Chun Leung, and Wa Yat Wu), who eagerly joined my research lab for an undergraduate research project. They were captivated by the fascinating phenomenon of droplet freezing and trampolining in a low-pressure environment, as showcased in a previous study (Nature, 527, 82-85, 2015). Serendipitously, Dr. Xiao Yan, an experienced young researcher working on thermofluid and heat transfer from the University of Illinois at Urbana-Champaign (UIUC), joined our lab, and I invited Dr. Yan to co-supervise the undergraduate team with the assistance from my master student Chun Yin Au (Samuel). These bright young minds have teamed up in my lab, and our collective journey has now settled into a cohesive phase.
Vaporization Momentum is the Key
Despite the challenges posed by the Covid lockdown, the team persevered, working tirelessly with limited access to the lab while donning masks. As their project progressed, intriguing differences emerged in their results, particularly regarding droplet compression, which deviated from the findings of the Nature paper. By carefully analysing the videos and repeating the experiments, we proposed an intriguing hypothesis—the vaporization process itself introduced a momentum to the droplets, termed vaporization momentum, which caused compression. This hypothesis contradicted the previously reported overpressure underneath the droplet on structured surfaces. Intrigued by this idea, we embarked on a deeper exploration of the phenomenon.
Decoupling the effects of overpressure and vaporization momentum proved to be a challenging task. We made significant efforts to differentiate these two mechanisms by quantifying the momentum (jumping velocity), examining the effects of surface structures by varying the length scale of structures, and developing comprehensive models to predict jumping velocity. Inspired by slippery surfaces used for antiscaling (ACS applied materials & interfaces, 12, 12054-12067, 2020), we adopted similar surfaces to eliminate overpressure completely. On these structure-free surfaces, we observed droplet sliding upon freezing, providing direct evidence for the existence of vaporization momentum. Our study emphasizes the fact that when a liquid undergoes intensive phase change, it is subjected to a force that will profoundly impact the transport dynamics.
Closure
Our journey, from a classroom lesson to laboratory and eventually to publication in Nature Communications, highlights the importance of openness, collaboration, and communication, especially during times of isolation in Covid pandemic. This publication is a cherished memory for our team, coinciding with significant milestones in our individual paths. The graduate students who participated in the research graduated in 2022, while our master student Samuel has embarked on his Ph.D. journey at the University of British Columbia, Canada. Dr. Xiao Yan has become a professor at Chongqing University, China, while I continue to work with new students here in Hong Kong, striving to ignite further advancements in the field of phase change and heat transfer. Just like a droplet freezes and then jumps, our brilliant minds have united and then traversed the globe.
Go check it out:
Yan, X., Au, S.C.Y., Chan, S.C. et al. Unraveling the role of vaporization momentum in self-jumping dynamics of freezing supercooled droplets at reduced pressures. Nat Commun 15, 1567 (2024). https://doi.org/10.1038/s41467-024-45928-2
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in