What Your Eyes Reveal About Brain Aging and Alzheimer’s Risk
Published in Healthcare & Nursing, Neuroscience, and Protocols & Methods
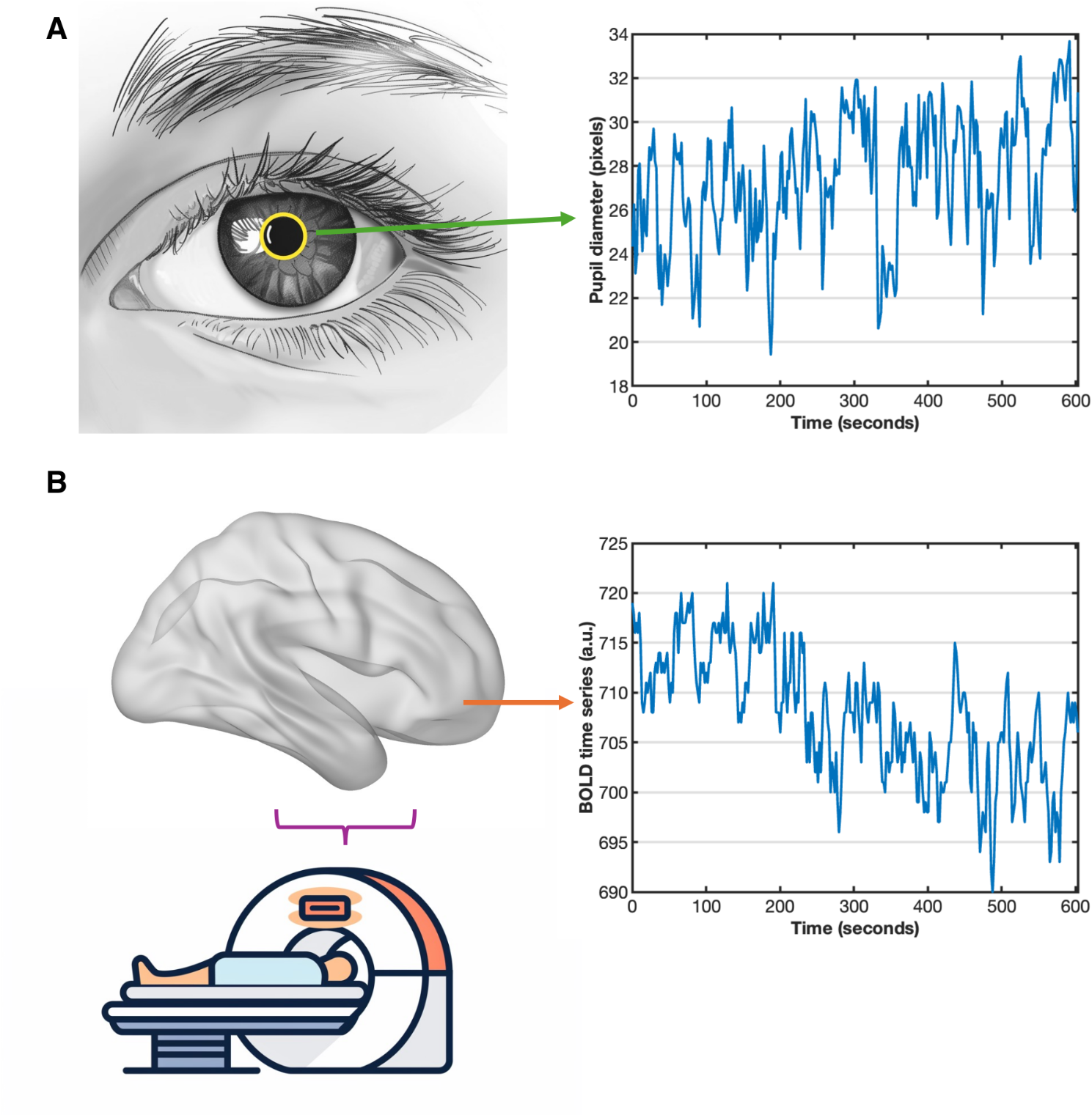
The pupil does more than respond to light—it also reflects activity in a small but powerful brain region involved in attention, memory, and Alzheimer’s disease. By tracking natural pupil changes during rest and linking them to brain scans, this study uncovers how brain networks shift with age. These insights suggest a simple, non-invasive way to study early brain changes long before symptoms appear.
Follow the Topic
Related Collections
With Collections, you can get published faster and increase your visibility.
New voices in biomarker research for neurodegenerative diseases
Detecting and monitoring the pathophysiology underlying neurodegenerative diseases remains a major challenge in ensuring treatment of the right individual with the right drug at the right time. While development and validation of biomarkers for Alzheimer’s disease (AD) have advanced rapidly, progress in other neurodegenerative disorders continues to lag despite recent breakthroughs. Representing this highly dynamic and fast-paced area of research, this special Collection gives early career researchers a voice to share some of the very latest in the field.
The Collection includes articles from early career delegates of the course “Biomarkers for Neurodegenerative Diseases”, jointly organised since 2018 by the University of Gothenburg, University College London, and the Barcelona Beta Research Center, to bring together world-leading scientists and brilliant young researchers to share and learn about the latest developments. Originally created to bridge gaps between neuroimaging and fluid biomarker research, it now supports the educational needs of the next generation of researchers.
A series of reviews covers new perspectives on the prominent role of neuroimaging in capturing the spatiotemporal patterns of pathophysiology across the Alzheimer’s disease spectrum, the current state of fluid biomarker research, and the integration of biomarkers into clinical practice, still one of the greatest challenges faced by scientists and clinicians alike. These are complemented by research articles on Stable Isotope Labelling Kinetics (SILK)-based profiling of biomarkers associated with neurodegenerative diseases in cerebrospinal fluid (CSF), and comparing tau biomarkers from positron emission tomography (PET) and blood plasma measures for predicting the clinico-biological progression of AD in asymptomatic individuals.
This Collection welcomes submission of original research articles and reviews from early career researchers. Topics of interest include, but are not limited to:
• Neuroimaging and fluid biomarkers
• Genetic and molecular markers
• Longitudinal and translational studies
• Multimodal integration in diagnosis and prognosis
• Biomarkers in non-Alzheimer’s neurodegenerative diseases
This Collection supports and amplifies research related to SDG 3, Good Health and Well-Being.
All submissions in this collection undergo the journal’s standard peer review process. Similarly, all manuscripts authored by a Guest Editor(s) will be handled by the Editor-in-Chief. As an open access publication, this journal levies an article processing fee (details here). We recognize that many key stakeholders may not have access to such resources and are committed to supporting participation in this issue wherever resources are a barrier. For more information about what support may be available, please visit OA funding and support, or email OAfundingpolicy@springernature.com or the Editor-in-Chief.
Publishing Model: Open Access
Deadline: Aug 21, 2026
Advances in Lewy Body Dementia Research
Lewy body dementia (LBD) remains one of the most challenging neurodegenerative disorders to diagnose and treat, making continued research essential. Its complex pathophysiology, often presenting alongside other neurodegenerative conditions, demands deeper investigation to clarify the mechanisms driving disease progression and symptom variability. Recent advances have enabled earlier detection and clinical trial activity in Lewy body dementia is increasing, especially in individuals with mild cognitive impairment. The emergence of seeding amplification assays presents a step-change in our ability to study alpha-synuclein pathology, a hallmark of LBD.
Ongoing research promises to improve diagnostic precision, inform targeted therapeutic strategies, and offer insights into the prodromal stages of the disease. A better grasp of co-pathology could also lead to the development of more representative disease models, capturing the multifaceted nature of LBD and its overlap with other neurodegenerative disorders. By expanding our understanding, we not only advance clinical care but also contribute meaningfully to the broader landscape of research in dementia pathophysiology.
This Collection welcomes original research articles and reviews. Topics of interest include, but are not limited to:
• Biomarkers in Lewy body dementia
• Prodromal disease, including mild cognitive impairment
• Disease pathophysiology
• Disease models of Lewy body dementia
• Treatment strategies and clinical trials
• Co-pathology with other neurodegenerative diseases
• Seeding amplification assays in research
This Collection supports and amplifies research related to SDG 3, Good Health and Well-Being.
All submissions in this collection undergo the journal’s standard peer review process. Similarly, all manuscripts authored by a Guest Editor(s) will be handled by the Editor-in-Chief. As an open access publication, this journal levies an article processing fee (details here). We recognize that many key stakeholders may not have access to such resources and are committed to supporting participation in this issue wherever resources are a barrier. For more information about what support may be available, please visit OA funding and support, or email OAfundingpolicy@springernature.com or the Editor-in-Chief.
Publishing Model: Open Access
Deadline: Jul 28, 2026

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in